Download the WhitePaper

Contraception Is Not Meeting The Needs Of People In The United States
A White Paper By The NewGen Contraception Project
FAQs
Acknowledgements:
The drafting of this white paper was a collaborative effort between NewGen Contraceptive Project (Sarah Cairns-Smith and Helen Jaffe) and two consultants (Chelsea B. Polis and Lucy Wilson). SCS, HJ and J. Joseph Speidel drafted the introduction and conclusion.
CBP drafted Sections II, III, and IV. LW drafted Sections V and (with SCS) VI. J. J. S. reviewed the entire document and made substantive comments and contributions to the final version of the white paper.
All final wording was determined by NewGen Contraceptive Project and J. Joseph Speidel.
We would also like to acknowledge the contributions of our NewGen research associates to this paper: Zoe Matticks, Alyssa Kulmer, Amy Liu, Chloe Diggs and Emily Thompson Glass.
Overview
-
Introduction: What is the White Paper Aiming To Do
-
Lays out the contraceptive options, a baseline to examine the degree to which contraceptives are meeting user needs and factors driving the gap between high use and high levels of unintended pregnancy
-
Look at the contraceptive experience from three perspectives
Section III – Views the key factors that influence contraceptive decision making
Section IV - Examines what drives contraceptive use and nonuse, continuation and discontinuation and the available evidence on contraceptive satisfaction levels
Section V - Takes the data presented in Sections II-IV from the perspective of underserved populations
Taken together sections II-V attempt to outline the current state of contraceptive usage and dis/satisfaction with existing options -
Switches focus from user needs to the state of the field of contraceptive R&D
-
Summarizes the major gaps in the current contraceptive options and makes a call for increased investment to create a new
generation of contraceptives -
Appendices A - F
-
Terms and Abbreviations
-
The references used in the main white paper
Abstract
This white paper describes why contraceptives are not currently meeting people’s needs in the United States. Women+ are experiencing high rates of contraceptive failure, unintended pregnancies, and many experience a high level of dissatisfaction with available methods. Men+ have few options. Yet the level of interest and investment in contraceptive research and development (R&D) is very low. This paper makes the case for more investment in contraceptive R&D - to improve our understanding of reproductive biology, to discover more contraceptive targets, to apply advanced science in the discovery of new product options, and to fund the testing of those options in clinical trials. Renewed interest and investment in contraception is essential for us to have a New Generation of Contraceptives and improve on our current experiences with contraception.
Section I: Introduction

This report documents why the array of contraceptive methods currently available to people in the United States is not meeting their needs. Evidence for this assertion includes the following: 1) Women+ are experiencing high rates of contraceptive failure and unintended pregnancies; 2) Survey research has established the desired characteristics of contraceptives and reveals a substantial level of dissatisfaction with available contraceptive methods; 3) The current array of contraceptive methods has clear gaps and therefore is not meeting the specific needs of many different groups of existing and potential users; and 4) The level of interest and investment in contraceptive research and development (R&D) is very low, in part because the extensive use and apparent diversity of existing options drive a mistaken perception that contraception is a “solved problem.”
For more on this topic, see also our commentary in Contraception, June 2024 (Cairns-Smith et al., 2024).
Section II
This section lays out the major contraceptive options and their relative popularity and effectiveness. This creates context from which to examine the degree to which contraceptives are meeting user needs and the factors driving the gap between high contraceptive use and high levels of unintended pregnancy.

Section II: Contraceptive options: The current state of contraceptive usage in the United States
Contraceptive failure and unintended pregnancies
Almost two thirds of women+ in the United States use contraception – often for decades (Daniels & Abma, 2020). Even so, nearly half (45%) of all pregnancies (about 2.5 million annually) are unintended and 42% of unintended pregnancies end in abortion (Guttmacher Institute, 2020b). Although many unintended pregnancies result from reasons such as lack of access to contraception, non-use, and improper use, many commonly used contraceptives fail, even when used correctly.
Data on the risk of pregnancy for each contraceptive method is shown in Figure 1. The most commonly used reversible contraceptive method, the combined oral contraceptive (COC) has a pregnancy rate of 7% under typical use conditions but only 0.3% a year when used perfectly in the first year of use (Hatcher et al., 2018). Similarly, the male condom has a failure rate of about 13% under typical use compared to 2% when used perfectly in the first year of use. The long-acting reversible contraceptive (LARC) methods (IUDs and implants), and the permanent methods (male and female sterilization) are much more reliable. They do not depend on conscientious behavior by their users and have pregnancy rates of 0.1% to 0.8%.
The unfortunate reality is that over many decades of typical use of currently available contraceptives there will be one or several unintended pregnancies. Even with perfect use of high efficacy methods there will be pregnancies. For example, according to a study by Tietze and Bongaarts, if all couples used a 90% effective contraceptive after having two children they would end up with a total of 2.84 pregnancies (Tietze & Bongaarts, 1975). They concluded that, “It is unlikely that any population has ever attained a low level of fertility (average births per woman of 2.2 or less) without the use of induced abortion, legal or illegal. More widespread and more effective use of contraception reduces the need for abortion; however, abortion is not likely to disappear at the levels of contraceptive effectiveness currently attained and attainable.” Given people today face almost the same array of contraceptive options as when Tietze and Bongaarts drew this conclusion, but access to abortion is being restricted in some areas, it is even more essential that we invest in new methods.
Figure 1: Contraceptive pregnancy rates and usage1
Adapted from Contraceptive Technology 22nd Edition
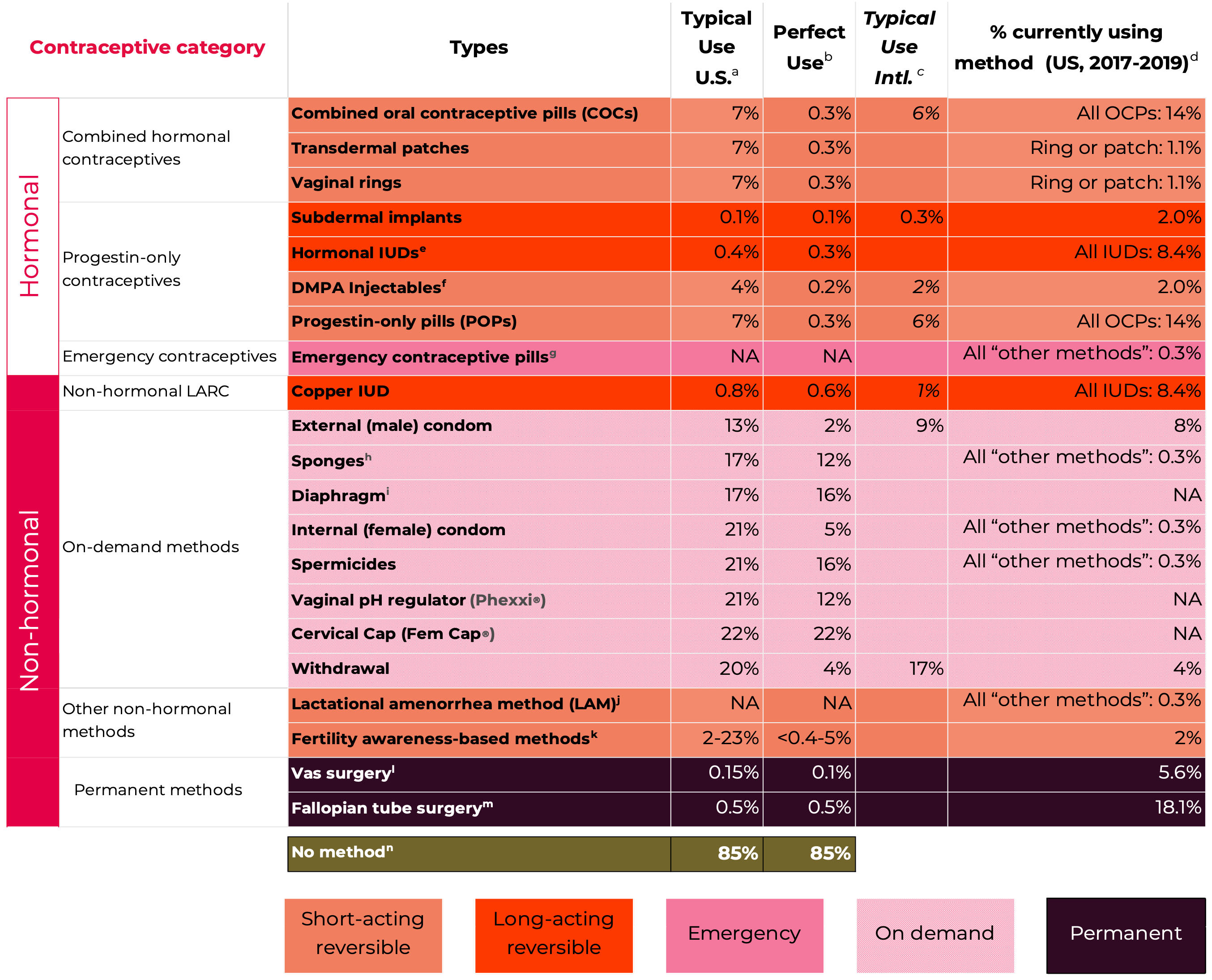
Notes
1 Pregnancy rates, table and notes from Contraceptive Technology, 22nd Edition (Bradley et al., 2023; Cason et al., 2023) with some adaptions – including inclusion of some data from Contraceptive Technology, 21st Edition (Hatcher et al., 2018).
a Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly) for the first year, the percentage who experience an accidental pregnancy if they do not stop use for any other reason. Most estimates in this column come from clinical data; (See (Bradley et al., 2023)).
b Among couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year of typical use if they do not stop use for any reason other than pregnancy. Estimates of the probability of pregnancy during the first year of typical use for withdrawal, the male condom, the pill, and Depo-Provera are taken from the 2006–2010 National Survey of Family Growth (NSFG) corrected for under-reporting of abortion. (See (Bradley et al., 2023)).
c Among couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any reason other than pregnancy. Estimates in this column are based on population-based Demographic and Health Survey data from 15 countries, not adjusted for under-reporting of abortion. All estimates in this column are calculated using life table data. (See (Bradley et al., 2023)).
d Data for percent distribution of women aged 15-49 by current contraceptive status, United States, 2017-2019 derived from: https://www.cdc.gov/nchs/data/databriefs/db388-tables-508.pdf#2, by most effective method reported.
e For details rates for specific LNG-releasing IUDs (See (Bradley et al., 2023)).
f Depot-medroxyprogesterone Acetate (DMPA, DepoProvera) injectables.
g Combined ECPs, levonorgestrel, ulipristal acetate:
If used after unprotected sexual intercourse, emergency contraceptive pills or insertion of a copper IUD reduce the risk of pregnancy substantially. However, due to methodological challenges in assessing EC effectiveness, estimates of failure rates are not included in the table. Use of emergency contraceptive pills or placement of an IUD after unprotected intercourse substantially reduces the risk of pregnancy. (See (Cason et al., 2023)).
h Estimates are for all sponge users. For nulliparous women, the perfect-use pregnancy rate is 9% and the typical use pregnancy rate is 14%. For parous women, the typical-use pregnancy rate is 27% and the perfect use pregnancy rate is 20%.
i With spermicidal cream or jelly.
j Lactational Amenorrhea Method: LAM is a highly effective, temporary method of contraception. However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches 6 months of age. (See (Cason et al., 2023)). 12-month pregnancy rates are not available for this method, which should be used for a maximum of 6 months.
k Multiple FABM methods with varying features and guidelines for attempting to predict the fertile window. Range based on Sensiplan (2% typical, 0.4% perfect use), Natural Cycles (7% perfect use), Clue (8% typical, 3% perfect use), Standard Days (13% typical, 5% perfect use), Billings (23% typical, 3% perfect use). All with U.S. typical use data. And Calendar rhythm (15% typical U.S., 19% typical International, NA perfect use). (See Chapter 15, Fertility Awareness-Based Methods, (Cason et al., 2023)).
l Vas surgery (vasectomy, male sterilization).
m Fallopian tube surgery (female sterilization).
n This estimate represents the percentage who would become pregnant within 1 year without using contraception. (See (Bradley et al., 2023)).
Contraceptives currently in use in the United States
Contraceptive products marketed in the United States fall into two major product classes - hormonal and non-hormonal, and four method groupings. These are:
- Short-acting reversible contraceptive methods (hormonal combined and progestin-only pills, patches, rings, injectables; and non-hormonal behavior-based methods such as Fertility awareness based-methods and lactational amenorrhea methods),
- Long-acting reversible contraceptive methods (implants and IUDs; and the non-hormonal copper IUD),
- On-demand options (spermicides and gels, diaphragms, sponges, male and female condoms, cervical caps, and emergency contraceptives may also be included in this class), and
- Permanent contraceptive methods for both men+ and women+.
As can be seen in Figure 2, 34.1% of women age 15-49 are using the highly effective permanent (sterilization) and long-acting reversible (LARC) contraceptive methods (IUDs and implants) and 31.2% are using one of the less-effective methods.
The less-effective methods include many varieties of combined hormonal contraceptives (CHCs): combined oral contraceptives (COCs) in pill form; transdermal contraceptive patches worn on the skin; and intravaginal contraceptive rings. They contain both estrogen and progestin, suppress ovulation and thicken cervical mucus, thereby reducing the chances of fertilization. Also among the less-effective methods are progestin-only contraceptives (POCs), progestin-only pills (POPs), and injectables (e.g. Depo- Provera®). POPs (often referred to as ‘mini-pills’) are taken orally every day, while injectables are delivered every three months.
The hormonal methods dominate the United States contraceptive market. The latest count was 285 individually marketed contraceptive products (Drugs.com, 2023). The vast majority are hormonals with variants on the same few ingredients in different dosage and delivery forms, with many fewer truly distinct products.
People who did not use a contraceptive method, or whose method failed can also use emergency contraceptive pills (ECPs) to prevent pregnancy. ECPs can be taken up to 5 days after unprotected sex, although taking the pills as soon as possible improves their effectiveness. IUDs can also be used as emergency contraception.
On-demand, or coitally dependent, methods have few medical contraindications. They may be appealing for people who have sexual intercourse infrequently but tend to require more user action for correct and consistent use, leading to lower typical-use effectiveness compared to hormonal or long-acting and permanent methods. Spermicides, internal (“female”) and external (“male”) condoms, and sponges are available over-the- counter, while pH-altering vaginal gels, diaphragms, and cervical caps require a prescription. Relative to the male-condom, none of these methods has so far had strong market uptake.
Other non-hormonal methods include fertility awareness-based methods (FABMs), Lactational Amenorrhea Method (LAM), and withdrawal (coitus interruptus). These methods have no side-effects, but their effectiveness is highly variable.
Figure 2: Current contraceptive status of U.S. women aged 15-49
From National Survey of Family Growth, 2017-2019
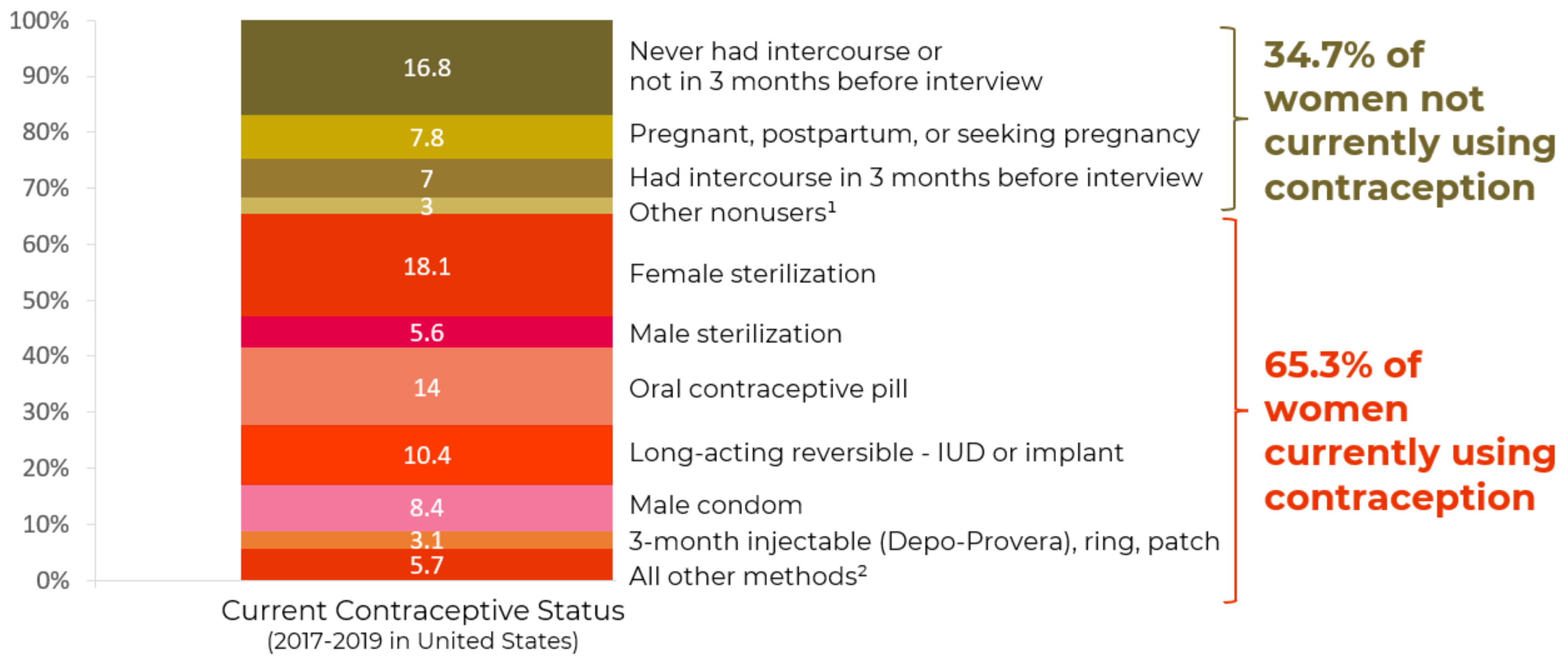
1Additional categories of nonusers, such as nonsurgical sterility, are shown in the accompanying data table: see source below
2Other methods grouped in this category are shown in the accompanying data table: see source below.
NOTES: Percentages may not add to 100 due to rounding. Women currently using more than one method are classified according to the most effective method they are using. Access data table for Figure 2 at National Center for Health Statistics, National Survey of Family Growth, 2017-2019: https://www.cdc.gov/nchs/products/databriefs/db388.htm.
Sections III-V
look at the Contraceptive experience from the perspective of individual users:
- Section III: focuses on decision-making
- Section IV: focuses on use/non-use, continuation/discontinuation, satisfaction/dissatisfaction
- Section V: focuses on populations that are underserved by the current method mix
Taken together sections II and III-V attempt to outline the current state of contraceptive usage and dis/satisfaction with existing options.

III: Decision-making
Section III - looks at the key factors that influence contraceptive decision-making for individual users.
Section III: Key factors influencing contraceptive decision-making
Many factors influence contraceptive method choices and satisfaction. The desired characteristics and decisions about their use are greatly influenced by an individual’s life circumstances; the characteristics of available methods; and the availability and quality of contraceptive information and services. These components vary over time, with changes potentially spurred by coitarche, relationship status transitions, changes in fertility desires, major reproductive events (e.g., birth, abortion, or miscarriage), or other life events.
A recent global systematic review of values and preferences for contraception assessed 423 articles from 93 countries around the world. The review found that values expressed by people choosing a contraceptive method centered on “themes of choice, ease of use, side effects, and effectiveness” and that “many users also considered factors such as cost, availability, interference in sex and partner relations, the effect of hormonal contraceptives on menstruation, and interactions with health workers” (Yeh et al., 2022).
Below, we briefly review these and other key factors pertinent to contraceptive selection (Coombe et al., 2016; Dam et al., 2022; Donnelly et al., 2014; Lessard et al., 2012; Madden et al., 2015; Ti et al., 2022), described in four broad categories1:
1. Real and perceived physical, mental, and sexual impacts,
2. Method- and use-related characteristics,
3. Life course and contextual factors,
4. Availability and quality of contraceptive information and services.
1. Real and perceived physical, mental, and sexual impacts
Effectiveness (and perceived effectiveness) at pregnancy prevention: A key factor that many people consider when choosing a contraceptive method(s) is how well it works to prevent pregnancy (see Figure 1) (Coombe et al., 2016; Donnelly et al., 2014; Lessard et al., 2012; Madden et al., 2015). While method-specific failure rates can be provided during contraceptive counseling, misunderstandings about contraceptive effectiveness persist (Meier et al., 2021). For example, a study of 500 non-pregnant women found that a majority (≥65%) over- estimated the effectiveness of condoms and oral contraceptives, particularly highly educated women (Kakaiya et al., 2017).
Health concerns, side effects, and side benefits: Health concerns and side effects are central to contraceptive decision-making for many individuals (Lessard et al., 2012; Madden et al., 2015). Real and perceived health impacts can deeply influence a person’s contraceptive risk- benefit calculation (Le Guen et al., 2021). A nationally representative web-based survey conducted in 2015 found that nearly half (49%) of respondents who ever used a prescription contraceptive method were concerned about side effects prior to starting, with the top two concerns being weight gain and mood swings (Nelson et al., 2018). Users of certain methods often report side effects (e.g., acne, breast tenderness, moodiness, headache, weight gain, etc.) or side benefits (e.g., reduced acne and cramping, protection against sexually transmitted infections, etc.) (Hatcher et al., 2018; Teal & Edelman, 2021). Several studies have investigated the impact of contraceptives on various psychological outcomes, such as anxiety, depression, memory issues, and more (Bitzer et al., 2018; Hatcher et al., 2018; Schaffir et al., 2016; Worly et al., 2018). Since many contraceptive studies are not placebo-controlled, understanding if certain impacts are attributable to the contraceptive method itself (vs. being due to other contextual factors, medications, health conditions, etc.) is complicated. Perceptions around contraceptive safety may be poorly aligned with current scientific evidence (for example, the inaccurate belief that oral contraceptive pills are more hazardous to a woman’s health than pregnancy is widespread (Kakaiya et al., 2017; Nelson et al., 2019)). Certain contraceptive methods are associated with rare but serious physical health risks (e.g., stroke, heart attack, blood clots, etc.), although beneficial associations also exist (e.g., reduction in rates of endometrial and cervical cancer) (Hatcher et al., 2018; Teal & Edelman, 2021).
Medical contraindications and drug-drug interactions: Contraceptive options may be limited by a person’s existing medical conditions or current use of certain medications (Marnach et al., 2020). While most contraceptive methods are considered safe for most users, particular conditions result in contraindications to use of specific contraceptive methods.
Contraceptive-induced menstrual bleeding changes: Hormonal contraceptive methods and IUDs can cause contraceptive-induced menstrual bleeding changes (including changes in bleeding duration, volume, frequency, and/or regularity or predictability). These changes can affect contraceptive users’ lives in positive and negative ways, often quite profoundly, and have been shown to be a key factor in contraceptive decision-making (Diedrich et al., 2015; Hoppes et al., 2022; Le Guen et al., 2021; Polis et al., 2018). Furthermore, different people interpret menstrual bleeding changes quite differently.
Fertility-related concerns: Although contraceptive use (regardless of duration) does not negatively impact future ability to get pregnant (fecundity), (Girum & Wasie, 2018; Mansour et al., 2011) some individuals fear that use of some reversible contraceptive methods will cause adverse impacts on their fertility (Le Guen et al., 2021). “Perceived infertility” may be associated with reduced motivation to use contraception consistently or at all, even if one is actually at risk of unintended pregnancy (Gemmill, 2018; Polis & Zabin, 2012).
Sexual acceptability: Concerns about reductions in sexual pleasure, enjoyment, spontaneity, stamina, or libido are related to avoidance of condoms and other contraceptives (Dalessandro et al., 2022; Higgins & Smith, 2016).
2. Method- and use-related characteristics
Use-related parameters: Some important use-related parameters include the duration of the fertility-regulating effect (Coombe et al., 2016; Madden et al., 2015) (and relatedly, the extent of user action required, including the frequency and ease of re-administration required and whether the method is coitally dependent (Ersek et al., 2011)), ease of use, ability to use the method discreetly, the extent of planning required to use the method, the experience of initiating the method (e.g., whether it involves pain during insertion or removal, or requires touching one’s genitals, etc. (Coombe et al., 2016)), the ability to use the method in conjunction with other products (e.g., tampons or other menstrual products) (Schnyer et al., 2019), and whether reliance on another person is required to either start and/or stop using the method.
Physical or functional characteristics of the method: For contraceptive methods which involve a physical product, that product’s physical characteristics (e.g., appearance, color, scent, size, taste, texture, flexibility, etc.) can induce emotional and psychological responses which may affect acceptability (Tolley et al., 2022; Zhao et al.,2022). Certain methods (e.g., gels, foams, spermicides, etc.), may be perceived as ‘messy’, and therefore impact the users experience (or anticipated experience). For some people, aspects such as the perceived ‘naturalness’ of the method (Le Guen et al., 2021; Woodsong et al., 2004), mechanism of action (Tong et al., 2022), route of administration (e.g., injection, oral, insertion, etc.), or mode of action (e.g., chemical, mechanical, surgical, behavioral) can also impact acceptability.
3. Life course and contextual factors
Life course factors: Where a person currently is within their reproductive journey can influence the contraceptive methods that are desirable and/or medically appropriate. Among people at risk for unintended pregnancy, age is strongly associated with stability of method use or type of method chosen (Daniels & Abma, 2020; Pazol et al., 2015, p. 20). For example, increasing age makes use of sterilization more likely and use of OCPs less likely (Daniels & Abma, 2020).
Contextual factors: An individual’s social circle may include intimate partner(s), friends, family members, healthcare providers, media sources, and more – each of which play an important role in shaping opinions about contraceptive methods (Yee & Simon, 2010), and in some cases, may also play a more direct role in shaping contraceptive choices. Several studies demonstrate that the importance of health care providers in these dynamics, while also acknowledging the importance of the role of peers and family members – but noting that they are often a source of negative or inaccurate information (Mahony et al., 2021; Yee & Simon, 2010). Beyond the influence of individual people within one’s social network, broader societal influences including religion (Srikanthan & Reid, 2008; Woodsong et al., 2004) and extent of acculturation into a given society (Gilliam et al., 2011) can also impact whether and which contraceptive methods an individual perceives as being potentially appropriate for their use.
4. Availability and quality of contraceptive information and services
Contraceptive counseling and other decision-support models: Many people rely on providers for assistance in making contraceptive decisions, and the content and quality of contraceptive counseling can impact uptake and satisfaction (Ali & Tran, 2022; Committee on New Frontiers in Contraceptive Research et al., 2004). A recently proposed definition of contraceptive counseling is “the exchange of information on contraceptive methods based on an assessment of the client’s needs, preferences, and lifestyle to support decision-making as per the client’s intentions. This includes the selection, discontinuation or switching of a contraceptive method. The key principles are based on: coercion-free and informed choice; neutral, understandable and evidence-based information; collaborative and confidential decision- making process; ensuring respectful care, dignity, and choice” (Ali & Tran, 2022). The American College of Obstetricians and Gynecologists (ACOG) recommends intentional application of a patient-centered reproductive-justice framework and use of a shared decision-making model of contraceptive counseling (American College of Obstetricians and Gynecologists (ACOG), 2022; Dehlendorf et al., 2017; Holt et al., 2020).
The 2016 U.S Selected Practice Recommendations for Contraceptive Use published by the CDC recommends that common side effects (e.g., unscheduled spotting or light bleeding, or heavy or prolonged menstrual bleeding especially during the first 3-6 months of use) should be discussed during contraceptive counseling and prior to, for example, insertion of the Copper-IUD (Curtis et al., 2016), as such counseling and reassurance that bleeding irregularities are not harmful may reduce method discontinuation.
Service availability and costs: Some individuals in the United States (and particularly populations that are already marginalized), face barriers to accessing contraceptive services, such as issues related to cost, stigma, or challenges in physically accessing services (Swan, 2021). For example, Power to Decide reports that more than 19 million women of reproductive age in the United States are in need of publicly-funded contraception but live in a contraceptive desert (i.e., lack reasonable access in their county to a health center that offers the full range of contraceptive methods) and 1,149,920 United States women in need live in counties without access to a single health center that provides the full range of methods (Contraceptive Deserts 2024 | Power to Decide, 2024). Furthermore, while most private health insurance plans in the United States are required to cover contraceptive methods, counseling, and services under the Affordable Care Act, 21% of privately insured women still paid some amount out-of-pocket for contraceptive care in 2020 (Frederiksen et al., 2021).
Contraceptive features desired by people in the United States
Many studies have examined which contraceptive attributes people in the United States report prioritizing in a contraceptive method.
- A 2010 survey by Lessard et al of 574 women seeking abortions in the United States suggested that the three features of contraceptive methods rated most important included: effectiveness (84%), lack of side effects (78%) and affordability (76%) (Lessard et al., 2012). This study identified substantial gaps between the contraceptive features participants desired and the features in currently available methods: for 91% of study participants, no existing method had all features rated extremely important, largely due to women wanting a highly effective method with few or no side effects. Methods involving the most features ranked extremely important were rings and sponges.
- Online cross-sectional surveys conducted in 2013 by Donnelly et al asked convenience samples of 417 women and 188 contraceptive care providers in the U.S. to rate the importance of 34 common questions when choosing (or for providers, discussing with a patient) a contraceptive method (Donnelly et al., 2014). Overall, the eight questions most frequently noted as important by women and/or providers related to: method safety, mechanism of action, mode of use, side effects, effectiveness with typical and perfect use, frequency of administration, and when it begins to prevent pregnancy.
- A 2016 systematic review by Coombe et al which assessed qualities of LARCs perceived to be desirable or undesirable by young women in developed countries identified as top qualities: no need for daily action, high efficacy, and long-term protection (Coombe et al., 2016). The review also found that while qualities perceived as undesirable for LARCs varied, the most commonly reported were irregular bleeding, painful insertion and removal, weight gain, and location in the body (Coombe et al., 2016).
Some studies examining the relative importance of contraceptive attributes also attempted to examine associations between a person’s stated attribute preferences and actual use of a contraceptive method (and the attributes of that method):
- A 2010-2011 study by Madden et al which surveyed 2590 women in Missouri reported that the contraceptive attributes most important when choosing a method were effectiveness and safety, followed by cost, whether the method is long-lasting and forgettable, health care providers’ recommendation, avoiding irregular bleeding, STI protection, and side effects (Madden et al., 2015). Every attribute participants were asked to rank was highly ranked by at least a few women – emphasizing the need for contraceptive options to meet diverse needs and preferences. Most women (69%) reported experiencing at least 1 side effect from a contraceptive method, and 65% of those women said the side effect was severe enough to cause discontinuation. Women who prioritized “long- lasting” or “forgettable” methods were more likely to choose IUDs, implants, injectables, rings, or patches compared to OCPs. Women who prioritized having regular monthly periods and avoiding irregular bleeding were more likely to choose OCPs than IUDs or implants.
- Marshall et al analyzed data from the 2009 National Survey of Reproductive and Contraceptive Knowledge, including from 715 women aged 18–29 who had ever used contraceptives (Marshall et al., 2016). Among seven contraceptive attributes, the largest proportion of respondents ranked these three as being extremely important: effectiveness at pregnancy prevention (79%), effectiveness at HIV/STI prevention (67%), and ease of use (49%). Interestingly, no statistically significant association existed between highly rating contraceptive effectiveness and actually using a highly effective method (sterilization, IUD, or implant). Conversely, rating “hormone-free” as highly important was associated with being less likely to use hormonal methods, and rating HIV/STI protection as highly important was associated with using condoms (either alone or as part of dual protection). Black and Hispanic women were far less likely (75% lower odds, and 78% lower odds, respectively) than White women to use a hormonal method, and were more than 3-4 times as likely to use less-effective contraceptive methods.
- Walker et al conducted a 2015-2016 survey of 814 sexually experienced girls and women aged 13-24 in family planning clinics in Northern California, and found that the three most highly-rated contraceptive features included: effectiveness (87%), safety (85%), and having few or no side effects (72%) (Walker et al., 2019). Age, race, and history of intimate partner violence or of an STI were associated with ratings. For example, younger women and Black, Hispanic, and Asian women were more likely than White women to prioritize STI prevention and privacy, and Black and Hispanic women were more likely to prioritize the ability to use a method without a doctor or clinic. When individuals were using a contraceptive method consistent with their preferences (on effectiveness, partner independence, and privacy), they were more likely to report contraceptive satisfaction. Interestingly, this study found no association between a strong desire for contraceptive effectiveness and current use of a highly effective method.
Prioritization of effectiveness doesn’t necessarily correlate with actual usage
While substantial variation in prioritization of contraceptive attributes exists across different studies, populations, and sub-groups, effectiveness was consistently identified as among the most important attributes. However, findings in multiple studies suggesting that stated prioritization of this attribute is not necessarily associated with actual use of a highly effective method (Samari et al., 2020). It is unclear why this apparent contradiction exists – it could be related to misunderstandings around the actual contraceptive effectiveness of various products, or to the importance of tradeoffs that contraceptive users must make when selecting an actual method – particularly when high effectiveness is often tied to characteristics that may be perceived as more invasive (i.e., requiring surgery or a device to be inserted into the body). The strong medical and public health emphasis on more highly effective methods sometimes overrides the recognition that less- effective methods which offer other attractive features may be preferred by some populations, perhaps particularly people of color, as demonstrated in several studies described above and below.
Some studies have examined why some users prefer to use less-effective methods. For example, Berglas and colleagues conducted in-depth interviews in 2016 with predominantly young Latina and African American women seeking emergency contraception, to better understand their contraceptive preferences (Berglas et al., 2021). They found that preferences centered around three main themes. The first theme was a preference for flexibility and spontaneity over continual contraceptive use. For example, some respondents preferred not to commit to using an ongoing method, and the perception of lower-efficacy methods being simpler to access and use. The second involved an emphasis on protecting their bodies from immediate side effects (e.g., weight changes, acne, depression, etc.) while also on protecting their future ability to become pregnant. The third theme involved their feelings on how well a method worked for them to prevent pregnancy. In some cases, this pertained to their clarity of understanding how the method could prevent pregnancy (for example, some felt a sense of security from clearly understanding how a condom can physically stop a sperm from meeting an egg), for others, widespread use of a given method encouraged a perception that the method must work well enough for so many people to use it.
In sum, contraceptive attributes prioritized by many United States contraceptive users across multiple studies include contraceptive effectiveness, lack of side effects (including irregular bleeding), safety, affordability, mechanism of action, ease of use, and duration of effectiveness, among others – although specifics vary by study and across population subgroups, including for minoritized women. There is a gap between the constellation of ideal contraceptive features and the array of existing methods, and data suggests that closing this gap could result in greater contraceptive satisfaction. However, it is essential to acknowledge that perhaps the most commonly-stated attribute – effectiveness – is not always reflected in the methods people actually choose to use – which may speak to the tradeoffs users must make in selecting an actual method, to misunderstandings about effectiveness, or to other factors.
Global systematic reviews suggest that counseling can play an important role in a user’s choice to use or not use a contraceptive method (Yeh et al., 2022), and so attention to the values and preferences of contraceptive providers – and whether or not these values and preferences are client-centered – is also an important component of ensuring that individuals ultimately can access methods with which they will be satisfied (Soin et al., 2022). Decision-making is also influenced by disinformation which is now potently amplified through social media, for instance there have been concerns about backlash against hormonal contraceptives based on unsubstantiated claims (Sloat, Sarah, 2022; Weber & Malhi, 2024).
1 1In aiming to provide a brief overview, we acknowledge that this list is not exhaustive, particularly for categories #3 and #4.
IV. Contraceptive use and non-use, continuation and discontinuation, satisfaction and dissatisfaction, in the United States
Section IV examines what drives contraceptive use and nonuse, continuation and discontinuation, and the available evidence on contraceptive satisfaction levels.
Section IV: Contraceptive use and non-use, continuation and discontinuation, satisfaction and dissatisfaction in the United States
Contraceptive use and non-use
Few studies in the United States have provided nationally representative information on the extent of, and characteristics associated with, contraceptive non-use among sexually active people capable of becoming pregnant but not seeking pregnancy. A study using National Survey of Family Growth data from 2011-2017 estimated that only 5.7% of women “at risk of unintended pregnancy”2 in the United States could reliably be considered contraceptive non-users (Frederiksen & Ahrens, 2020) at a given point in time. Due to variation in methodological approaches, other studies in the United States have suggested higher estimates, ranging up to 16.5% (Fowler et al., 2019; Kavanaugh & Pliskin, 2020; Mosher et al., 2015; Pazol et al., 2015). Studies in other countries suggest much higher rates of contraceptive non-use; for example, an analysis of data from 47 low- and middle-income countries suggested an average rate of contraceptive non-use of 41% (Moreira et al., 2019). In the United States, contraceptive nonuse has largely remained steady for at least the last 15 years (except among people aged 20-24, among whom nonuse increased between 2014 (10%) to 2016 (17.3%), despite increased access to more highly effective contraceptive methods (Kavanaugh & Pliskin, 2020).
Contraceptive non-use is higher in older women (Frost et al., 2007; Godfrey et al., 2016; Pazol et al., 2015; J. Wu et al., 2008), particularly those who report having difficulty achieving pregnancy or who intend to have children in the future (Pazol et al., 2015). For teens, discontinuing contraception due to dissatisfaction is also associated with contraceptive non-use (Pazol et al., 2015). Associations between contraceptive non-use and various other factors have been reported, including: being Black (Dehlendorf et al., 2014; Frost et al., 2007; Grady et al., 2015; J. Wu et al., 2008) and particularly young and Black (Dehlendorf et al., 2014), being uninsured or on Medicaid (Mosher et al., 2015; J. Wu et al., 2008), being obese (Nguyen et al., 2018), being concerned about parents finding out about contraceptive use (Iuliano et al., 2006), having infrequent intercourse (Frost et al., 2007; J. Wu et al., 2008), not currently being in a relationship (Frost et al., 2007), cohabitating (vs. being married) (Mosher et al., 2015), having perceived fertility problems (Borrero et al., 2015; Mosher et al., 2015), and holding fatalistic attitudes towards pregnancy (Frost et al., 2007; R. K. Jones, 2018). A curvilinear association has been observed between education and contraceptive non-use, with non-use more likely both at lowest and highest levels of education (Frost et al., 2007; Mosher et al., 2015; J. Wu et al., 2008).
Key reasons stated for contraceptive non-use among women at risk of (or who experienced) unintended pregnancy included: “do not expect to have sex”, “do not think you can get pregnant”, “don’t really mind if you get pregnant”, and “worried about the side effects of birth control” (Frederiksen & Ahrens, 2020; Mosher et al., 2015). A study that provided information on the relative importance of these reasons suggested that (based on nationally representative data from 2002-2010, collected among women who had an unintended pregnancy in the three years before the interview) the most frequently stated reason for contraceptive non-use was “did not think I could get pregnant” (41%) – which varied by race, with 50% of Hispanic women and only 29% of Black women stating this reason (Mosher et al., 2015). In this study, only 10% of contraceptive non-users stated that the reason was being worried about side effects (Mosher et al., 2015), though this was among the top reasons for non-use in more recent nationally representative studies (Frederiksen & Ahrens, 2020).
Non-use of one’s preferred contraceptive method is common. A nationally representative study using data from 2015-2017 found that 22% of reproductive age women at risk of unintended pregnancy in the United States would have preferred using a different contraceptive method if cost were not a factor (K. L. Burke et al., 2020).
Contraceptive continuation and discontinuation
Contraceptive discontinuation is sometimes viewed as a proxy for understanding contraceptive acceptability and satisfaction, as method- related reasons (such as concerns about a method’s side effects) play a key role in decisions to switch or discontinue contraception (Simmons et al., 2019). Ideally, contraceptive counseling can adequately prepare a user to anticipate and manage certain side effects, and inadequate counseling may contribute to contraceptive discontinuation if unanticipated side effects occur (Danna et al., 2021). Studies do suggest that method dissatisfaction is associated with method discontinuation (Frost et al., 2007) or higher intention to switch (Steinberg et al., 2021). However, some method discontinuation is due to other contextual factors (e.g., a change in fertility intentions or in sexual partners, a new medical contraindication, etc.). Furthermore, some users select shorter-acting methods because they do not anticipate long-term use (Steinberg et al., 2021) (e.g., women who wanted to become pregnant within five years were less likely to accept free IUDs or implants (Geist et al., 2020)). Shorter-acting methods may therefore appear to have greater discontinuation, even if method satisfaction was high. Still, other method characteristics may also impact discontinuation rates – for example, discontinuation of implants and IUDs generally require care from a provider, unlike discontinuation of most other methods – making continuation a passive event.
For reasons such as these, researchers have cautioned against interpreting use or continuation (sustained use) of a contraceptive method as an unambiguous indication of method satisfaction, as it may reflect merely tolerating the method (versus being satisfied with it), encountering challenges in stopping the method, or a lack of acceptable alternative method options (Dehlendorf et al., 2018; A. Glasier, 2010; Severy & Newcomer, 2005). Literature on contraceptive discontinuation has also been critiqued for failing to adequately capture and report voices of women and subjective experiences (Inoue et al., 2015).
A 2002 nationally representative study of women in the United States specified reasons for contraceptive discontinuation, which avoids some (but not all) of the measurement challenges noted above. This study found that nearly half (46%) of all users of reversible contraception reported at some point having discontinued at least one reversible method because they were unsatisfied with it (Moreau et al., 2007a). More than half (52%) of women who ever used a diaphragm or cervical cap discontinued due to dissatisfaction (Moreau et al., 2007a). The corresponding proportion for other methods included: sponge (48%), Depo-Provera® or Norplant® (42%), spermicidal foam, suppository, gel (39%), IUD (36%), oral contraceptive pills (OCPs, 29%), female condoms (24%), patch (20%), fertility awareness-based methods (15%), withdrawal (13%), male condoms (12%) (Moreau et al., 2007a). Specific reasons for discontinuation were available only for users of OCPs, Depo-Provera®, Norplant® and condoms; a majority (>65%) of hormonal method users discontinued due to side effects (Moreau et al., 2007a) (as also observed in other studies (Littlejohn, 2012)). The second most common reason was contraceptive-induced menstrual bleeding changes (particularly for Depo- Provera®) (Moreau et al., 2007a), which other studies have also highlighted as a key determinant of contraceptive discontinuation (Inoue et al., 2015; Polis et al., 2018). Condom discontinuation generally related to dissatisfaction for a sexual partner (39%) or a decrease in sexual pleasure (38%) (Moreau et al., 2007a). Discontinuation of hormonal contraception due to dissatisfaction has also been observed to be more likely among less educated women, with no differences observed by race or ethnicity (Littlejohn, 2012).
While contraceptive acceptability does not appear to have a singular, agreed upon definition in the field, Elias and Coggins wrote in 2001 that for a method to be acceptable “a potential user must fully understand the potential benefits of using it, the elements of correct use, its potential side effects, and alternative methods and be willing and able to consistently apply such knowledge to the use of the technology in everyday life” (Elias & Coggins, 2001). They suggest that studying contraceptive acceptability is important because it ultimately plays a key role in the method’s typical use effectiveness (Elias & Coggins, 2001). Heise noted in 1997 that beyond simply focusing on product attributes, “feminists have argued that ‘acceptability’ must be viewed as a complex interplay between a woman, a technology, and a service delivery environment” (Heise, 1997).
Contraceptive satisfaction and dissatisfaction
An important factor to consider in interpreting results from studies of contraceptive satisfaction is whether the study includes only current users of the method, or includes both current and former users of the method, as the latter group may include those who discontinued the method due to low satisfaction since not including them may artificially inflate apparent satisfaction measures (Moreau et al., 2007a).
Our review found only one nationally representative study of contraceptive satisfaction among women in the United States has been published since 2000. A telephone survey of adult women at risk for unintended pregnancy in 2004 measured satisfaction of women with their current method (if they were using one) or with the method they had most recently discontinued (if they were not currently using one) (Frost et al., 2007). Even among women who had continued to use the same method during the last year, only about 71% reported being very satisfied with their method (Frost et al., 2007); another 25% were “somewhat” satisfied and 4% reported being either neutral or dissatisfied (Frost et al., 2007). Among those who had switched methods or discontinued altogether, the percent of women who reported being very satisfied with their method ranged from 44%-52%, while feeling neutral or dissatisfied ranged from 15%-24% (Polis, 2022)
Limited information on contraceptive satisfaction is available from other key studies. For example, a longitudinal study (2015-2017) in Salt Lake County, Utah collected data from new contraceptive clients aged 18-45 who received their desired method at no cost (Kramer et al., 2022). At three months, 52% of participants were “completely satisfied” with their chosen method, and another 31% were “somewhat satisfied”. While 4% reported being “neither satisfied nor dissatisfied,” 7% reported being “somewhat dissatisfied” and another 6% were classified as “completely dissatisfied”. Satisfaction may have been influenced by the opportunity for participants to choose their preferred method at no cost, as well as by characteristics of the analytic sample (e.g., nearly 70% used a LARC, sample did not include adolescents, etc.).
One compelling study among women in North Carolina found that trying a LARC, even when not initially seeking one, resulted in high satisfaction. Among of women initially seeking short-acting methods but voluntarily randomized to receive LARC, 71% reported being “happy” with their LARC at the end of two years, 73% said they would use the method in the future, and 81% reported that they would recommend a friend or relative to try the method (Hubacher et al., 2018).
A study of a diverse sample of young women aged 13-24 recruited from family planning clinics in Northern California in 2015-2016 suggested that contraceptive satisfaction was higher when their current method matched preferences on any of three contraceptive attributes: effectiveness, ability to use a method independently of a partner’s knowledge, and privacy (Walker et al., 2019).
In sum, in the United States, a relatively small proportion of people (likely about 6%) but ranging up to 17% in some studies) are at risk of unintended pregnancy but not using a contraceptive method, though this may be higher within certain sub-populations. Key reasons for such non-use are not solely limited to concerns about side effects (which relate to method satisfaction), but also pertain to perceptions around the likelihood of pregnancy or attitudes towards the idea of pregnancy (which do not likely relate to method satisfaction). Those using contraception are not always using their preferred method (sometimes due to cost-related reasons), which is a barrier to contraceptive satisfaction.
The relationship between contraceptive discontinuation and contraceptive satisfaction is complex, and continuation should not necessarily be interpreted as satisfaction and vice versa. Indeed, some contraceptive continuers may be tolerating while dissatisfied with their method. A 2002 nationally representative study found that nearly half (46%) of all users reported at some point having discontinued at least one reversible method because they were unsatisfied with it (Moreau et al., 2007a). The study suggested that discontinuation specifically due to dissatisfaction was lowest for male condoms, withdrawal, fertility awareness-based methods, and the patch, while discontinuation due to dissatisfaction was highest for cervical cap/diaphragms, sponges, and Depo-Provera®.
Research that aims to measure contraceptive acceptability, including satisfaction, has faced challenges in conceptual clarity, measurement, and interpretation (Heise, 1997), and how acceptability is measured often depends on whether the product is currently hypothetical, in clinical trials, or available on the market. Quantitative studies from 2000 or later assessing multiple methods available in the general population found that 44-71% of contraceptive users reported being “very satisfied” with their method (depending to some extent on whether use was current or discontinued). While another 25-31% report being “somewhat satisfied.”
There is clearly substantial room for improvement in contraceptive satisfaction in the United States population. In these studies, certain characteristics were associated with contraceptive satisfaction, including method attributes (e.g., ease of use, perceived effectiveness, side effects, privacy), method accessibility (e.g., cost), individual characteristics (e.g., age, race/ethnicity, partnership status, health status, recency of sex), and counseling experiences (e.g., experiencing shared decision-making, not feeling the provider had a preferred method).
Some scholars focused on low- and middle-income countries argue that “the current definition of unmet need undercounts the number of women with a true unmet need for contraception as it misses the many women who are using a method that does not meet their preferences” and call for adding satisfaction questions in Demographic and Health surveys (Rominski & Stephenson, 2019). Better nationally representative estimates of contraceptive satisfaction among current and former users – ideally also including men+ – would be helpful to guide research aiming for improved contraceptives.
2 Defined by Frederiksen and Ahrens as: “not currently pregnant or seeking pregnancy, were not (or their partner was not) infecund for non-contraceptive reasons, did not expect a birth in the next 2 years, were not using contraception at the time of the interview, and either had sex that month or last had sex within the prior year while not pregnant and did not use contraception at that time.”
V. Categories of contraceptive users that are most underserved by the current method mix
Section V looks at the data presented in Sections II-IV from the perspective of underserved populations.
Taken together sections II-V attempt to outline the current state of contraceptive usage and dis/satisfaction with existing options.
Section V: Many Categories of Contraceptive Users Are Poorly Served by Current Options
The current array of contraceptive technologies has limited suitability or other drawbacks given the specific needs of many different groups of users and potential users. Sub-populations of the underserved include: groups with health concerns; groups with behavioral needs; and certain demographic and socioeconomic groups.
Groups with health concerns
People with concerns about side effects: This was the second most commonly cited reason for non-use of contraception, reported by 21% of women at risk of unintended pregnancy who were not using a contraceptive method in the 2011-2017 NSFG (Frederiksen & Ahrens, 2020)
People with contraindications to specific methods: The CDC’s United States Medical Eligibility Criteria for Contraceptive Use (MEC) (Curtis et al., 2016) outlines these medical conditions, risk factors, and contraindications to specific contraceptive methods. The largest number of conditions and risk factors are associated with the estrogen found in combined hormonal contraception (CHC). In a nationally representative sample of women in the United States, 16% of fecund women aged 20-51 years had contraindications to CHC (Shortridge & Miller, 2007a).
Table 1: Medical Eligibility Criteria for Contraceptive use - MEC 3 and MEC 4 conditions by type of contraceptive method
| Method | MEC 3 Conditions | MEC 4 Conditions |
|---|---|---|
| All combined hormonal contraceptives (CHCs) |
|
|
| Initiation of CHCs |
| |
| All progestin-only methods |
|
|
| Continuation of progestin-only methods |
| |
| DMPA |
| |
| Implants |
| |
| POPs |
| |
| Oral contraceptives (POP or COC) |
| |
| Initiation of IUDs (copper or hormonal) |
|
|
Source: CDC U.S. MEC 2016 (Curtis et al., 2016)
*Indicates that the condition is listed on the table more than once, or for more than one method type.
This table does not include all contraceptive methods. For the methods not listed here, there are either few or no conditions for which use is contraindicated.
MEC category definitions:(WHO, 2015)
MEC 1: A condition for which there is no restriction for the use of the contraceptive method
MEC 2: A condition where the advantages of using the method generally outweigh the theoretical or proven risks
MEC 3: A condition where the theoretical or proven risks usually outweigh the advantages of using the method
MEC 4: A condition which represents an unacceptable health risk if the contraceptive method is used
As there are many contraindications, some of which are fairly prevalent in the United States population, it would be helpful to have an estimate of the size of the population that faces contraindications. Determining this figure, however, is challenging. Estimates have only been made for contraindications to combined hormonal contraception (CHC), and most of those analyses were done to understand the risks of self-screening for over-the-counter access to oral contraceptive pills. In the only nationally representative sample of women in the United States, 16% of fecund women aged 20-51 years had contraindications to CHC (Shortridge & Miller, 2007b). In other studies, the reported estimates for prevalence of CHC contraindicated conditions varied from 2.4% - 19%, with differences in how the study populations were defined and the criteria used to define those with a CHC contraindication (Coleman- Minahan et al., 2021; Lauring et al., 2016; Xu et al., 2014). Two of those studies asked participants to self-report if they had a contraindicated condition (Coleman-Minahan et al., 2021; Lauring et al., 2016), which was found to over- estimate the actual percentage of participants with a contraindication (Xu et al., 2014).
Women+ who are postpartum: This group is at particular risk for unintended pregnancy. An analysis of 2006-2010 NSFG data found that at least 70% of pregnancies that occurred within one year of a previous birth were unintended. (White et al., 2015). Underestimating fecundability and not anticipating sexual activity in the postpartum period are common reasons for not contracepting (White et al., 2015). Potential contraceptive users also face limited options3 (Curtis et al., 2016). Postpartum use of estrogen-containing methods is contraindicated, because there is an increased risk of deep vein thrombosis, as well as a very small risk that estrogen can affect milk supply during breastfeeding (A. Glasier et al., 2019). Perceptions around the risks of using hormonal contraception while breastfeeding may also limit contraceptive use beyond the relatively short window for which it is medically contraindicated (Cwiak et al., 2004).
A greater variety of contraceptive products is needed during the postpartum period, including more highly effective, reversible methods that are non- hormonal, and particularly non-estrogenic. Methods like the progesterone vaginal ring Progering® have been developed to extend the contraceptive effectiveness of lactational amenorrhea among breastfeeding women, though this method is currently available only in a limited number of countries (Population Council, 2015).
People who are also concerned about HIV and other STIs: Most Americans will contract a sexually transmitted infection (STI) at some point in their life (Chesson et al., 2014), and the incidence of gonorrhea, chlamydia, and syphilis are rising in the United States (Centers for Disease Control and Prevention, 2022). Dual protection options that can simultaneously prevent both unintended pregnancy and the transmission of STIs, including HIV are needed. At present, condoms are the only multipurpose prevention technology (MPT) option.
Groups with behavioral needs
People who have infrequent sex: In an analysis of the 2011-2017 NSFG, not expecting to have sex was cited by nearly 14% of respondents as the reason for contraceptive non-use among those at risk of pregnancy (Frederiksen & Ahrens, 2020). (This figure was 24% for women who had experienced an unplanned birth in the previous three years in the 2006-2010 NSFG.) Nationally representative data from 2016/18 found that approximately 8% of women aged 18-44 years had sex only once or twice per year, and a further 26% had sex only 1-3 times per month (Ueda et al., 2020). People who have infrequent sex would benefit from the development of additional methods of ‘on-demand’ contraception, i.e., methods that can be used at or around the time of intercourse. On-demand options currently available are limited to emergency contraceptive pills and less-effective methods, condoms (under typical use), withdrawal, cervical barrier methods, and spermicides (Cahill & Blumenthal, 2018), as well as the recently marketed vaginal gel, Phexxi®.
People who are reluctant to interact with the health care system: Many people in the United States are reluctant to interact with the health care system, especially for reproductive health care. It includes members of racial and ethnic minority groups, for whom historic reproductive injustice as well as current experiences of racism may reduce willingness to seek products that require medical intervention (van Ryn et al., 2011). Those who identify or present as a sexual or gender minority, and youth may also be hesitant to seek health care. Reluctant groups may also include people living with a disability and people in larger bodies. As with people with limited geographic access to a health care facility and those seeking more convenience in accessing care, this population would benefit from additional self- administered options, including methods that can be started and stopped by the user.
People who want to use contraception discreetly: The ability to use a contraceptive method discreetly, without a partner’s or parents’ knowledge, is a factor in contraceptive choice and satisfaction, especially for young people (Coombe et al., 2016; Walker et al., 2019). People who lack autonomy in their relationship or are at risk of domestic violence may also want to use contraception discreetly. A variety of products is needed that do not require interaction with the health system, lead to fewer side effects (such as a lack of contraceptive-induced menstrual bleeding changes) and are not likely to be found or felt by the user’s partner, family, or friends.
Demographic and socioeconomic groups that are underserved
Men+ and others who desire male-based contraception: Currently, the overwhelming burden of reproductive control is placed on women+, as the contraceptive options available to men+ are limited. Male options include vasectomy, condoms, and withdrawal (Hatcher et al., 2018). Fertility awareness-based methods require the cooperation of both partners. (Peragallo Urrutia & Polis, 2019). Even though use of these methods results in 29% of all contraceptive use in the United States (Guttmacher Institute, 2021), men+ are often not considered to be users of contraception. More male contraceptive options are not only important to expand the options available for men+ but would also benefit couples for whom the female options are limited by acceptability, accessibility, and safety. Market research sponsored by the Male Contraception Initiative investigated interest from men+ in using male methods, they found that 8.1 million men between the ages of 15-44 in the United States would be very likely to use a new male method and 5.6 million men would be somewhat likely to use a new male method (Interest Among United States Men for New Male Contraceptive Options: Consumer Research Study, 2019). And a survey of women in three countries (China, South Africa, and Scotland) found that more than 70% of women would be willing to rely on male hormonal contraception if it were to become available (A. F. Glasier et al., 2000). A modeling study estimated that, even under conservative assumptions about uptake, introducing a male pill or reversible vas occlusion would decrease unintended pregnancies by 3.5% to 5.2% in the United States (Dorman et al., 2018).
Black, Hispanic, and people of color: Multiple nationally representative studies have reported that Black women (and potentially other people of color) at risk of unintended pregnancy are less likely to use contraception than White women (Frost et al., 2007; Grady et al., 2015; Guttmacher Institute, 2020b; J. Jones et al., 2012; R. K. Jones, 2018; J. Wu et al., 2008). An analysis of the 2006-2010 NSFG found that, among women using contraception, Black and Hispanic women were about half as likely as White women to use a highly- or moderately effective method (Dehlendorf et al., 2014). A similar result was found in a review of a 2009 nationally representative survey - non- White women were found to be 3-5 times as likely to use less-effective methods than their White peers (Marshall et al., 2016). Another reported that the racial disparity in use of highly effective contraception may be increasing over time (Jacobs & Stanfors, 2013), although this finding should not necessarily be interpreted as a negative outcome, if people are making informed trade-offs and increasingly using their preferred method.
These findings may contribute to the fact that in the 2011 NSFG, the rate of unintended pregnancies among Black women was more than double, and for Hispanic women nearly double, that of White women (79, 58, and 33 unintended pregnancies per 1,000 females aged 15-44) (Finer & Zolna, 2016; Guttmacher Institute, 2020b). In addition, Black women had an abortion rate more than three times that of White women (23.8 and 6.6 abortions per 1,000 women aged 15-44 years) in the CDC’s abortion surveillance data for 2019 (Kortsmit, 2021). As abortion access continues to be constrained as a result of the June 2022 Dobbs v. Jackson Women's Health Organization Supreme Court Decision, the impact will be particularly impactful for Black and other people of color.
Black, Hispanic, and other people of color make up nearly 40% of the United States population (N. Jones et al., 2021), and are also more likely to fall into other underserved groups, such as those with less geographic access (Kreitzer et al., 2021) and with limited resources and concerns about cost (Artiga et al., 2021; Kavanaugh et al., 2022). The intersectionality of race and ethnicity with geographic access to health care, income and education levels, and insurance status, combined with racism and discrimination in the health care system, have adversely affected the health of Black, Hispanic, Indigenous, and other people of color (Sutton et al., 2021, Roberts, 2014, (Gurr, 2011; Hooton, 2005 ). This sometimes results in a reluctance to engage with the health care system.
In considering why Black, Hispanic, and other people of color are more likely to be underserved by the current method mix, the reasons are likely to include concerns about safety and side effects, differential access to care, differential experiences with the health care system, differential preferences for various method attributes including greater interest in STI protection and on-demand options (Dehlendorf et al., 2014; Lessard et al., 2012). In the 2002- 2010 NSFG, analysis of data from respondents with a recent unintended birth showed Black women were more likely than White women to report concerns about side effects and not expecting to have sex among their reasons for non-use (Mosher et al., 2015), however, other analyses of dissatisfaction and discontinuation did not find differences in reasons among racial and ethnic groups (Littlejohn, 2012). Multivariate analysis of desired attributes of contraceptive products has found differences between people of color and White people, including greater interest in products that the user can stop using at any time, are only used with intercourse, and will not affect menstrual periods among people of color (Jackson et al., 2016). Black and Hispanic women were also more likely to report that STI protection, control over whether and when to use the method, and rapid return to fertility were important. Among currently available methods, these features match closely to less-effective methods. These product attributes, especially those allowing greater control over use, as well as those related to side effects and return to fertility, may result from concerns about reproductive coercion and mistreatment and/or neglect from the health care system.
As people of color have multiple reasons for contraceptive nonuse, dissatisfaction, and discontinuation, developing a range of new products that offer a variety of product attributes would better serve this population.
People who identify as LGBTQ* or gender expansive: Non-heterosexual- identifying women, who are estimated to represent 5.6% of adult women in the United States (J. M. Jones, 2021) have rates of unintended pregnancy that are higher than those among heterosexual-identifying cis-women, especially among younger women (less than 25 years of age) (Charlton et al., 2020; Reynolds et al., 2021; Stoffel et al., 2017).
Estimates published in June 2022 based on data from the CDC’s Behavioral Risk Factor Surveillance System and Youth Risk Behavior Surveillance System indicate that more than 1.6 million adults and youth identify as transgender or gender non-conforming, or 0.6% of the population aged 13 years and older (Brown, 2022; J. M. Jones, 2021). Youth are much more likely to identify as non- cisgender: 1.4% of youth aged 13-17 years (Herman et al., 2022). While it is commonly believed that gender-affirming testosterone use is sufficient as a contraceptive, multiple studies have reported pregnancies in people who were using (or recently used) testosterone (Light et al., 2018; Moseson et al., 2021). As testosterone use during pregnancy is contraindicated because of risks to the developing fetus, individuals who are at risk of becoming pregnant and using testosterone should be advised to use highly effective birth control (World Professional Association for Transgender Health (WPATH), 2012).
Among participants in the PRIDE Study, a national, online, longitudinal cohort study of sexual and gender minority people, 186 (11%) wanted a future pregnancy, and 275 (16%) were unsure; 182 (11%) felt at risk for an unintended pregnancy (Moseson et al., 2021). People who identify as LGBTQ* or gender expansive may not be using, or not consistently using, contraception for many of the same reasons as their heterosexual- and cisgender-identifying peers. However, their contraceptive decision-making may also reflect the challenges that they have faced or expect to face in seeking health care, especially reproductive health care, and whether or not they are counseled on contraception (Stoffel et al., 2017). For transgender individuals, it may also include concerns about how side effects will affect their gender expression and identity, and the lack of information about the contraceptive effects of testosterone and the interactions between testosterone and contraception (Agénor et al., 2020; Bonnington et al., 2020; Krempasky et al., 2020). Products that would better meet the needs of these populations include those that require less interaction with the health system and a greater diversity of hormonal and non-hormonal options that would enable users to find a method that helps them affirm their gender identity without interactions with testosterone.
Those with limited resources and/or concerns about cost: Even though the Affordable Care Act (ACA) mandates that private insurers cover FDA- approved contraceptive methods with no out-of-pocket costs, there were still estimated to be more than 20.6 million women in the United States in need of publicly funded contraceptive services in 2016 (Frost et al., 2019). As such, the cost of contraceptive methods remains a barrier to use.
A review of the 2015 – 2019 NSFG data found that, if cost were not an issue, 23% of low-income female contraceptive users would use a different method, and 39% of low-income nonusers would use a method (Kavanaugh et al., 2022). Another study, using the 2015-2017 NSFG and looking at women at risk of unintended pregnancy, found that 22% of them would have preferred using a different contraceptive method if cost were not a factor (K. L. Burke et al., 2020).
Insurance status and income level: Insurance status and income level may also affect contraceptive use, but there are discrepancies in the findings related to this topic. For instance, among women at risk of unintended pregnancy, two multivariate analyses of NSFG data found that insurance status, but not income, was significantly associated with contraceptive non- use (Kavanaugh et al., 2022; J. Wu et al., 2008). A third analysis found insurance status to be significant, but did not report on income level (Mosher et al., 2015). Two others found neither to be significant after adjustments (Frederiksen & Ahrens, 2020; Grady et al., 2015). A sixth, which looked at the likelihood of using a preferred contraceptive method among women at risk of unintended pregnancy, found that it increased along with income level, but did not vary by insurance status (K. L. Burke et al., 2020). Uninsured women were also found to have higher odds of using a less-effective method than their insured counterparts (Marshall et al., 2016). While programmatic interventions and industry reforms may more quickly address cost concerns, modifications to existing methods to reduce cost, and a wider array of lower- priced generic and biosimilar brands could also help to meet the contraceptive preferences of uninsured and low-income people in the United States
People who have geographic access barriers: Having access to a wide range of contraceptive methods requires living within geographic proximity to a health care facility that offers contraception. According to Power to Decide, more than 19 million women of reproductive age in the United States are in need of publicly funded contraception and live in “contraceptive deserts” (counties without an adequate number of health centers that offer the full range of reversible contraceptive methods) and 1,149,920 United States women in need live in counties without access to a single health center that provides the full range of methods (Contraceptive Deserts 2024 | Power to Decide, 2024).
Another analysis, looking at data from 14 states and using different definitions and analysis methods, estimated that between 17% and 53% of each analyzed state’s population lived in a contraceptive desert. This analysis also found that racial minorities were overrepresented in contraceptive deserts (Kreitzer et al., 2021). The United States clearly needs more health care facilities and providers that offer contraception, especially in rural and low-income areas. The challenge of contraceptive deserts may also be addressed, at least in part, by having a broader range of contraceptive options that can be initiated, maintained over time, and/or removed without having to see a health care provider or visit a health facility.
Young people: Young people may also be underserved. For example, an analysis of the 2015-17 NSFG found that young women (aged 15-24) were more likely to report cost barriers to preferred contraceptive use compared to older women (K. L. Burke et al., 2020). While there are not contraindications specific to age (Curtis et al., 2016), providers are often reluctant to recommend certain methods to young people. Permanent methods are also generally not available to young people and those that do undergo sterilization at a relatively early age have a higher chance of regret (Danvers & Evans, 2022). In addition, the ability to access reproductive health care confidentially is especially important to adolescents and other young people (Lehrer et al., 2007). These concerns may limit a young person’s willingness to use methods that require interaction with a health care provider (Fuentes et al., 2018; R. K. Jones et al., 2005). Analysis of global data has also shown that younger populations experience much higher contraceptive failure rates, especially for user-dependent methods like pills, condoms, and withdrawal (Bradley et al., 2019). As such, young people may prefer contraceptive options that do not require interaction with a health care provider, can be used discreetly, offer dual protection, and are less user-dependent.
People older than 40 years: People who are approaching the end of their reproductive lives are also underserved by the current contraceptive options. While overall contraceptive use increases with age (Daniels & Abma, 2020), non-use of contraception among women at risk for unintended pregnancy may increase with age (Frost et al., 2007; Godfrey et al., 2016; Pazol et al., 2015). In a multivariate analysis of the 2002 NSFG among women who were at risk of an unintended pregnancy, women older than 40 had six times the odds of contraceptive nonuse compared to younger women (J. Wu et al., 2008). Unintended pregnancy rates are consistent with those among women of other ages, with estimates that 48% of pregnancies among women aged 40- 44 are unintended (Johnson-Mallard et al., 2017). Pregnancy also becomes more dangerous with age (Van Heertum & Liu, 2017). No contraceptive method is contraindicated based on age alone, but certain conditions are more common among older people, such as hypertension and venous thrombosis (Curtis et al., 2016; Miller et al., 2018; Van Heertum & Liu, 2017). As such, older women as a group have fewer contraceptive options. Reasons for non-use among older women are not well-studied. While perceived subfecundity is likely a primary reason (which providers might be able to address through education and counseling), other potential reasons include the increasing prevalence of contraindications and other health concerns (J. Wu et al., 2008). As such, older people would likely benefit from a greater diversity of methods that are highly effective and non-estrogenic.
Other potentially underserved groups
For people in larger bodies, an analysis of the 2006-2013 NSFG found that women with a BMI more than 35 kg/m2 and at risk of an unintended pregnancy were less likely to be using contraception than their lower BMI peers (Nguyen et al., 2018). Being “overweight” or “obese” does not result in any MEC 3 or 4 contraindications, but there is some evidence that emergency contraceptive pills may be less effective for those with BMIs more than 30 kg/m2 (Curtis et al., 2016; Ramanadhan et al., 2020). While people in larger bodies represent more than half of United States women of reproductive age (Hales et al., 2018; National Center for Health Statistics., 2019), there is limited socio-behavioral research on contraceptive use among people in larger bodies, so little is known about contraceptive decision-making and satisfaction among this population (Boyce & Neiterman, 2021). As such it is difficult to determine if new contraceptive methods would help meet the contraceptive needs of this growing population group.
For people living with disabilities and people at high risk of a medically complicated pregnancy, the little evidence we found indicated that these groups are potentially underserved (Cwiak, 2020; Stransky et al., 2021; Verlenden et al., 2019; J. P. Wu et al., 2017). Their reasons for contraceptive non-use, however, are either already addressed above (e.g., having a contraindication) or could not be addressed by new product options (e.g., limited provider knowledge about the population group). Some nationally representative studies identified those with less education as more likely to be non-users of contraception (Frost et al., 2007; J. Wu et al., 2008), while others did not find a significant association (Frederiksen & Ahrens, 2020). For this population group, reasons for non-use that might be addressed by new methods were not articulated in the literature.
In sum, the evidence on underserved populations and their reasons for nonuse, dissatisfaction, and discontinuation points to several product attributes that should be considered in developing new contraceptive methods. These product attributes include:
- High effectiveness
- Can be used “on demand”
- Minimal side effects and contraindications
- Health benefits
- Require minimal interaction with the health system
- Offer dual protection from unintended pregnancy and STIs
- Can be used discreetly
- Are affordable
- Are for use by men+
It should be noted that these attributes are also relevant to those who would not be considered underserved by current methods. In today’s method mix there are unavoidable trade-offs between many of these attributes leading to suboptimal choices for users.
3 Conversely, consistent breastfeeding during the first 6 months postpartum opens up the option of the Lactational Amenorrhea Method (LAM), if the user is amenorrheic.
Section VI
The state of the field
Section VI switches focus from user needs and looks at the state of the contraceptive research & development field and how well positioned it is to address these user needs.
Appendix E includes a brief review of products in the current pipeline.

Section VI: The state of the contraceptive R&D field
Context
Ongoing improvements in the current generation of contraceptives have been critical in advancing contraceptive effectiveness, safety, and/or acceptability, especially of hormonal methods. We have seen important product advances in dosing and delivery formats and the introduction of several generations of novel estrogens and progestins. We have also seen the combination of the IUD and progestins to create the hormonal IUD. But in contrast to this important, but relatively narrow, range of progress in contraception, we are seeing new products launched in other medical areas that represent extraordinary leaps of innovation. These innovations have delivered new classes of drugs such as monoclonal therapies, antibody-drug conjugates, and gene-editors/modulators/silencers such as RNAi and more personalized medicines. Even more traditional drug classes have benefited from approaches such as genetic analysis, gene knock-outs, novel screening and library techniques, and advanced assays during R&D. Such new approaches and new classes of drugs are only just beginning to shape the contraceptive product development.
In the last decade, there has been increased interest in new contraceptive development, with several calls for a contraceptive revolution (Anderson, 2019; Chamberlain et al., 2020; Haddad, Townsend, et al., 2021; Johnston & Kopf, 2023; Reproductive Blueprint, 2019; Speidel et al., 2020). There are also many new groups, including several small startups and their investors that have a growing interest in the field. Several male methods are also in development, with four clinical trials ongoing4. For female methods, however, much of the funding and work remains focused on making improvements to hormonal and other existing methods, such as changes to the dose, delivery method, and/or progestin, rather than developing methods with novel mechanisms of action. The pipeline for innovative products, especially highly effective, reversible, and non-hormonal products for women+, is slim.
For more on this topic, see also our commentary in Contraception, June 2024 (Cairns-Smith et al., 2024).
The R&D process
The development of a truly new and improved drug or device starts with investment in basic science. In contraception we are seeing exciting product concepts emerge from basic science, but it will take even greater investment in research to better understand reproductive biology and physiology and identify more places on the reproductive pathway to target for intervention.
Target identification (largely in academic labs) and validation (in academic, biopharma and non-profit developer labs) is followed by the identification and optimization of compounds or device prototypes that interact with those targets/opportunities for intervention.
The drug and device preclinical and clinical development programs that follow these research phases are long and costly processes. Many promising approaches need to be pursued to yield a successful product, as it is only from clinical trials, many years after the initial scientific discoveries, that we understand whether new products meet the required safety and efficacy standards for approval. Since contraceptives are given to healthy individuals over potentially a long time, the safety standards are even higher for this therapeutic area. Only 13% of the drugs that enter clinical testing get to commercialization (IQVIA, 2022) with clinical testing and approval taking around 10.5 years on average (BIO Industry Organization, 2022). Even getting to clinical testing is a major milestone, many more compounds fail earlier making drug R&D a highly risky endeavor. The long timelines involved for novel drugs are also financially challenging since companies have to invest far ahead of being able to recoup their investment. The median cost of bringing a single new drug to market is complex to calculate but many estimates fall in the range of $1-2+ billion, including accounting for failures (R&D in Pharma, 2021; Schlander et al., 2021; Wouters et al., 2020).
Contraceptive R&D funding landscape
The most recent G-FINDER data on contraceptive5 R&D funding indicates total global funding of approximately $149 million for 2022 (Policy Cures Research, 2024). This represents a slight increase from 2021 but a decrease from the two prior years, when the estimated annual funding was $168 million, but it remains above the level of funding in 2018 (Table 2). By funder, the largest decrease has come from aggregate industry, decreasing from nearly $52 million in 2018-19 to only $19 million in 2022. Industry has gone from being the largest funder, to the third largest, trailing the Bill & Melinda Gates Foundation and the United States National Institutes of Health. Over a few years the Bill & Melinda Gates Foundation has over doubled its investments in contraceptive R&D, with funding of $55 million in 2020 and 2021 and $66 million in 2022. Increases within the “other” category in 2021 are mostly attributed to the Population Council, which spent more than $10 million on its contraceptive R&D work in that year, a significant increase from prior years during which it spent between $3-3.5 million. In 2022, public funding from the United States government (NIH and USAID) plus Bill & Melinda Gates Foundation accounted for 84% of all spending. It should be noted that spend by start-ups is not well-captured by the G-FINDER methodology. However, at current levels of activity, such spend would not substantially increase these figures.
Table 2: Contraceptive R&D spending by funder – 2018-2022
Source: G-FINDER data portal(Policy Cures Research, 2024) – extracted April 2024. Rounding may affect totals. These numbers are estimates and may be refined over time as G-FINDER receives updated information.
Compared to previous decades, contraceptive R&D funding by the public sector has significantly declined. It has been estimated that the US NIH, USAID, and World Health Organization (WHO) provided approximately $104 million in funding each year during the 1970s, and $68 million in funding annually during the late 2000s (both figures inflation adjusted to 2020 United States dollars) (Thompson & Speidel, 2014). Within the last five years, US NIH and USAID spending was never higher than $57 million (with no reported spending by WHO).
Compared to other therapeutic areas, the level of overall funding, and of private sector funding in particular is very modest (Chamberlain et al., 2020; Speidel et al., 2020). Pharma companies typically reinvest around 20% of sales revenue in R&D for new products, whereas in contraception this figure is around 2% (Chamberlain et al., 2020). The contraceptive field, in addition to having relatively low investment, also has a different pattern of investment. In most therapeutic areas, public, and to a much lesser extent philanthropic, sources fund academic groups to conduct the basic biology that generates new ideas for drug/device intervention points. Then the private sector drives the discovery of potential drugs and prototypes, conducts pre-clinical and clinical testing, and leads manufacturing and marketing.
In contrast to other therapeutic areas, for contraception, academic institutions, along with non-profit product development partners (such as Population Council, FHI 360, CONRAD, PATH, and others), lead much of the basic science, preclinical work, and clinical trials, mostly with support from public and philanthropic funders. Private sector involvement comes in much later than in most other therapeutic areas with corporate partners stepping in once other funders have significantly de-risked the potential products. Most of the contraceptive methods available in the United States today were developed with substantial public and philanthropic dollars (including the first implant, the copper and hormonal IUDs, and the levonorgestrel and ulipristal acetate ECPs) (Thompson & Speidel, 2014). It seems likely that these cross-sector partnerships will continue to be essential to continued innovation and progress in contraceptive R&D going forward (Committee on New Frontiers in Contraceptive Research et al., 2004; Speidel et al., 2020).
Public sector: Between the US NIH and USAID, the United States government is, and has long been, a major player in contraceptive R&D globally (Policy Cures Research, 2024; Thompson & Speidel, 2014). Within the NIH, the National Institute for Child Health and Human Development (NICHD) funds a substantial amount of the basic research on reproduction, while also funding early, pre-clinical, and clinical research on contraceptive methods. A unique asset that the NICHD supports is the Contraceptive Clinical Trials Network, a network of highly qualified trial sites with the capacity to conduct Phase I, II, and III trials according to Good Clinical Practice standards, alongside a centralized statistical analysis and coordination center. NICHD primarily funds universities and other academic institutions, either for basic and early research or as clinical trial sites, with some industry recipients at the discovery and preclinical stages (Policy Cures Research, 2024). The focus of NICHD’s funding in recent years has been to support research on non-hormonal approaches for both men+ and women+.
USAID has had a role in many of the contraceptive methods on the market today. While it continues to be an important player, its financial support is less central than it was in the past. USAID’s focus is on developing new methods as well as improving on existing contraceptive methods designed for use by women+ in low- and middle-income countries (LMICs). For much of the 2000s and early 2010s, USAID’s budget for contraceptive R&D was around $10-12 million annually (Committee on New Frontiers in Contraceptive Research et al., 2004; Thompson & Speidel, 2014). USAID primarily funds non- profit product development partners, rather than universities and other academic institutions. Its projects focus on low-risk later stage work, including clinical research, post-registration studies, and introduction support. Despite its focus on LMICs, USAID-supported products are generally also marketed in high-income countries as the products need regulatory approval from the United States Food and Drug Authority (FDA) or another stringent regulatory authority to be purchased and distributed through USAID and other public sector purchasing programs for LMIC distribution.
Recent announcements from the White House on a Women’s Health Research Initiative offer potential for increases in funding for contraception in the United States (The White House, 2023b).
The WHO was an important funder in the past, but has moved away from funding contraceptive R&D. Besides the United States, a few other national governments provide funding, but the amounts are negligible compared to United States funding. The largest reported non-U.S. funding comes from the European Commission, which has funded between $1.2-1.5 million in 2020- 2021, and then from the French government, which funded just less than $1 million in 2018 and 2020 (Policy Cures Research, 2024). In late 2023 the German government added male and female contraception to a series of areas able to access a ~60 million euro funding pool, so it is likely Germany will become a more significant funder in the future (Szent-Ivanyi, 2023).
Philanthropic sector: Philanthropists and foundations have been supporting contraceptive development since the 1950s when an heiress, Katherine McCormick helped to fund the development of the oral contraceptive (Dhont, 2010). The Ford, Hewlett, Rockefeller, and Andrew W. Mellon Foundations were also engaged in contraceptive R&D efforts in its early days, but have long since exited the field (Committee on New Frontiers in Contraceptive Research et al., 2004; Thompson & Speidel, 2014). While several foundations have stepped in during recent decades, including the Susan T. Buffett, Hewlett, Packard, and Tara Health Foundations, the Bill & Melinda Gates Foundation is by far the biggest contributor (Speidel et al., 2020).
The Bill & Melinda Gates Foundation began its involvement in contraceptive R&D around 2008, and at the 2012 London Summit on Family Planning, made a public pledge to increase work in this area. It funds industry partners (mainly small biotech/startup companies) and academic institutions in the United States and abroad, as well as non-profit product development partners. As with USAID, their focus is on developing new and improved contraceptive options for use in LMICs. According to a recent strategy document, they are increasing their work in contraceptive R&D, which is already evident in their increased funding in the last few years (The Bill & Melinda Gates Foundation, 2021). The Bill & Melinda Gates Foundation is currently the largest single funder of contraceptive R&D, having surpassed the United States government. To date, most of the foundation’s funding has been on female, hormonal methods and platform technologies to build new tools for the field.
Private sector: In the 1960’s, several large pharmaceutical companies were engaged in contraceptive R&D efforts, including the Searle, Schering, Organon, Upjohn and later Wyeth, Pfizer, Merck (known as MSD outside the United States), and Bayer AG (Committee on New Frontiers in Contraceptive Research et al., 2004; Dhont, 2010). Today, there are fewer of these large companies, and fewer still are engaged in contraceptive R&D. Their investments in contraceptive R&D have ebbed (Barot, 2013b; Speidel et al., 2020) even while total pharmaceutical investment in R&D increased well over ten-fold (including adjusting for inflation) in two decades (R&D in Pharma, 2021). One reason is that the last decades have seen a number of mergers involving contraceptive players. Post-merger, contraception, which has several highly effective and safe products and a low base of basic biological research from which to generate new technologies, can find it hard to compete with other therapeutic areas in priority and investment debates. This has resulted in the closing of contraceptive and women’s health programs at many companies (Barot, 2013b; Burger, Ludwig & Weiss, Patricia, 2023; Speidel et al., 2020; Thompson & Speidel, 2014).
Embedded in this disinvestment is likely the perception that contraceptive R&D will have a low return on investment compared to other therapeutic areas (Thompson & Speidel, 2014). Rather than develop novel methods, pharma companies have opted for simple adaptations to existing methods, such as reducing dosage of hormones and improving delivery, as these are comparatively low cost and low risk. These modifications can have important patient benefits but they also help companies maintain high margins by extending their patent protection and keeping ahead of generic competition (Barot, 2013b; Speidel et al., 2020).
This is common practice - a recent estimate suggests that novel drugs (new molecular entities) account for only 36% of major company product launches (Frank & Hannick, 2022). But this practice is particularly dominant in contraception and over time the lack of investment in novel products can be seen not just in the paucity of new molecular entities in contraception but in the fact that hormonal contraceptives, despite being highly prescribed, are currently close to the bottom of major United States drug spending categories at 0.01% of annual spend ($5.5 billion) compared with 13% of annual spend for categories such as oncology, diabetes or immunology in 2019 (Aitken et al., 2020). The higher spend in these other therapeutic areas reflects the high numbers of novel products in those categories which can command higher prices.
Although the retreat of large pharma from contraceptive R&D on new molecular entities is concerning, there is also a scaling back of early-stage discovery and research work in other therapeutic areas. Only 10% of all drugs entering phase 3 originated in the largest companies and emerging biotech companies accounted for over 70% of the total R&D pipeline by 2018 (Aitken, Murray et al., 2023). These small entrepreneurial companies and their investors are beginning to recognize the market opportunities in contraception and are pursuing R&D efforts. Industry efforts might be boosted by investment in accelerator programs (Johnston & Kopf, 2023) which can enhance target identification and validation efforts, a crucial first step in the commercial R&D process and create important bridges between academic and industrial activities.
Companies such as Contraline, YourChoice Therapeutics, Evofem, Yaso Therapeutics, Cirqle, and others, are either focused exclusively on contraceptive products or have innovative compounds or delivery systems for which they are pursuing contraceptive indications. These companies are funded by a mix of private capital and philanthropic investments. Venture capitalists have begun investing in contraceptive R&D, most notably RH Capital which has an explicit focus on supporting new contraceptive development. Other VC and start-up accelerator programs supporting contraception include Amboy Street Ventures, Adjuvant Capital, the Founders Fund, the Y Combinator, and others. (Investments by these groups are not included in the spending dollars reported above.)
In July 2022, Cirqle Biomedical, developer of a non-hormonal on-demand contraceptive, announced it had entered into a research collaboration and licensing agreement with Organon (Organon, 2022). Bayer and Organon have also partnered with Daré Bioscience, a publicly traded biopharma company with several contraceptive products in its pipeline (Daré Bioscience, 2023). And in late 2022, Bayer and the Bill & Melinda Gates Foundation announced a $24 million partnership to support lead identification and pre-clinical work on non-hormonal female approaches (Bayer AG, 2022). However, in early 2023, Bayer announced that it would be tailing off internal research in women’s health, a disappointing development given so few larger pharma players in the field (Burger, Ludwig & Weiss, Patricia, 2023).
The foreign private sector is also engaged in contraceptive R&D. There are several India-based companies manufacturing generic and/or biosimilar approaches to products marketed by big pharma, and some of these companies are also innovating on their own. In November 2022, Pregna International announced it had received WHO/UNFPA prequalification for its copper-IUD packaged with a unique inserter for postpartum use (Taparia, 2022). China and Indonesia are also home to many contraceptive developers and manufacturers.
A snapshot of select examples from the female and male contraceptive pipelines is given in Figure 3a and 3b below.
For fuller details on the pipeline see Appendix E.
Figure 3a: Female Contraceptive R&D Pipeline
Select examples
Figure 3b: Male Contraceptive R&D Pipeline
Select examples
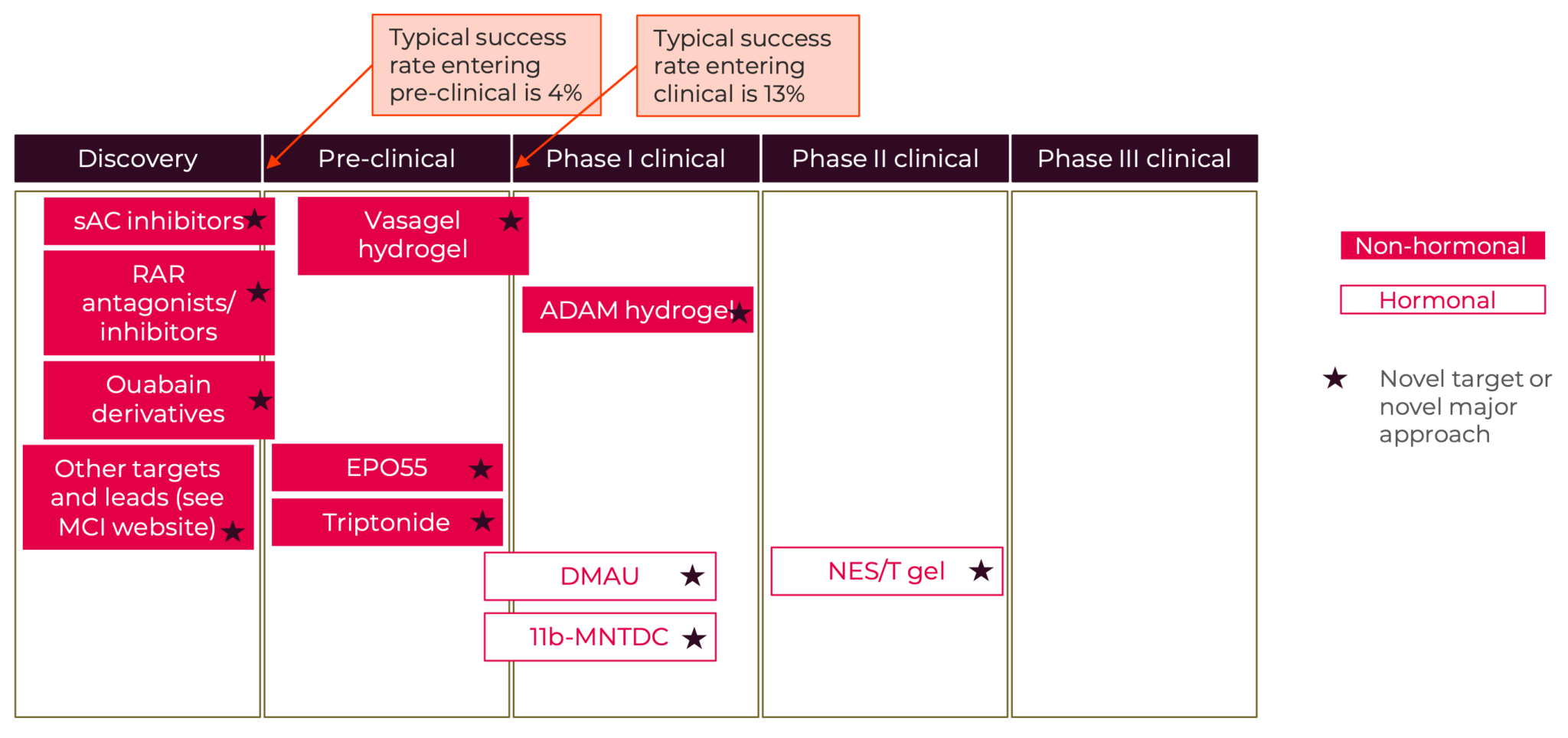
Other challenges facing contraceptive R&D
While the lack of funding at all stages from basic science to clinical development is probably the biggest challenge to bringing new contraceptive products to market, there are other barriers:
Bridging the gap from pre-clinical to clinical is a challenge common to all areas of drug development. These “translational” challenges include issues around FDA-required safety and toxicity studies, scaling manufacturing, and transitioning between the partners involved in the pre-clinical stages (typically academic institutions and small biotech) to those involved in the later clinical stages (typically large pharmaceutical companies) (Callahan et al., 2020; Committee on New Frontiers in Contraceptive Research et al., 2004; Haddad, Townsend, et al., 2021).
Product developers also face regulatory challenges, including decisions about clinical trial design, and long-term safety studies given the continual use of many methods. Guidance from the FDA on regulatory requirements for female non-hormonal and male methods is lacking, given the scarcity of these methods progressing through clinical trials, making clinical trial design even more challenging (Callahan et al., 2020; Haddad, Townsend, et al., 2021).
Pricing and reimbursement challenges can stifle innovation. Given the huge R&D investment costs required to discover and test new molecular entities and medical devices, a cornerstone of the innovation system is the patent protection that enables innovators to recoup investment costs with high initial product prices. To harness the extraordinary R&D financing capacity of the private sector, new products will almost certainly have to come with higher pricing than contraceptives that have been marketed for decades. Novel products need to find a supportive pricing and reimbursement environment. But the high prices needed to recoup R&D investments create a challenge because lower income or underinsured populations, often those most in need of wider choices, may struggle to pay for the newest products. So access strategies need to go hand-in-hand with novel product launch so that the widest possible populations benefit.
Payers such as insurers also have strong incentives, given the base of genericized products in place, to push for minimal use of more expensive novel products. While the Affordable Care Act has protections for contraceptives that should support reimbursement, there are many reports of this not occurring (Fey, Rachel & McDonald-Mosley, Raegan, 2022). The Biden administration has taken steps to address this but it is a key issue to monitor because a weak reimbursement environment would considerably reduce the incentives for companies to invest in contraceptive R&D (HHS, DOL, and Treasury Issue Guidance Regarding Birth Control Coverage, 2022; The White House, 2023a) https://www.dol.gov/sites/dolgov/files/ebsa/laws- and-regulations/laws/affordable-care-act/for-employers-and-advisers/letter- from-secretaries-becerra-yellen-and-walsh-on-the-aca-contraceptive- coverage-requirement.pdf.
Uncertain market size and demand is a challenge in this field because there is an existing base of products with proven efficacy and strong safety profiles. One common concern with regard to market demand is the possibility that companies may believe a new improved contraceptive will cannibalize the market for existing methods that require relatively little ongoing investment. It should be noted that this concern is now relevant to only the few remaining players in the field and new entrants wouldn’t have cannibalization concerns, though would need to believe in strong market demand.
High levels of unintended pregnancy, indicators of dissatisfaction and significant gaps in the method mix all point to the need for new methods, and the blockbuster success of the Mirena® hormonal IUD and rapid gains of market share by Lo Loestrin® (Chamberlain et al., 2020), strongly indicate untapped demand. But an innovator may have little sense of whether people using existing methods would be likely to switch if alternatives were available. This makes it difficult to forecast demand for the new product. Some of this uncertainty might be addressed by more detailed consumer-focused research on satisfaction with existing products.
There is also the possibility of litigation. Several high-profile and costly lawsuits have occurred related to safety and side effects of newer contraceptives (Barot, 2013b). Contraception is a preventative modality used by healthy individuals, this raises the bar on product safety and so the risk of product litigation.
Reputational /political concerns may occur because contraceptives are a controversial topic. Catholic doctrine has opposed the use of “artificial” contraception and abortion. Various anti-abortion groups have falsely called many current contraceptives, including IUDs, emergency contraceptives, and oral contraceptives, abortifacients. This is not in keeping with the science relating to mechanism of action. Misinformation and disinformation are always concerns with health products, but the politicization of contraception poses additional risks (Weber & Malhi, 2024).
Sexism may lead to lower recognition of the importance of having broader contraceptive options given most of the users of contraception have been women+, and many of those making investment decisions are men+. Even if this were to impact a single step in the investment chain, given each step is interlinked, the impacts could be considerable. For example, lack of early science investment reduces the substrate for product developers, lack of venture funding stifles early-stage product development and lack of pharma or device company interest closes off funding and co-investment for late- stage development.
The case for expanding investment in contraceptive R&D
Unfortunately, investment levels in contraceptive research and development (R&D) seem to reflect the “solved problem” perception. Despite considerable scientific advances in other therapeutic areas and product gaps in contraception, the R&D field is failing to innovate towards the goal of a new generation of contraceptives. A review of the United States clinical trials registry (clinicaltrials.gov) revealed only 12 active phase 1-3 clinical trials of contraceptive methods as of December 2022 (Home - ClinicalTrials.Gov, As accessed). By comparison, in 2022, oncology started over 2000 trials6; neurology over 600; and ophthalmology over 120, and 67% of all drugs launched in 2022 were first-in-class (Aitken et al., 2023).
Development of contraceptive products that have attributes desired by potential users and meet the needs of various underserved populations could bring the rate of unintended pregnancies down. The current array of contraceptive options is aging and current levels of investment in contraceptive R&D is inadequate to the task of developing new products. Most of the current contraceptives have their scientific origin in the 50s, 60s and 70s with innovation since then largely focused on improving on earlier breakthroughs.
Increased investment in contraceptive R&D should focus on products for women+ for several reasons. Women+ are likely to continue to carry a disproportionate contraceptive burden. Given the existing efforts in male contraception, a novel male option is likely to be approved within a decade. And there are promising but neglected novel approaches to develop improved female contraceptives.
In contrast to most therapeutic areas, public and philanthropic funders have been dominant in contraceptive R&D for novel products, with many of these players having a strong focus on developing products for low- and middle-income countries (Barot, 2013b; Thompson & Speidel, 2014). Increased investment from multiple actors focused on high-income markets could develop new and improved contraceptive options that would benefit both the United States and people globally.
Given the history of lower private sector engagement in contraceptive R&D than in other therapeutic classes, and the strong track record of government- and philanthropy-sponsored R&D activities, it may be tempting to believe that the future of contraceptive R&D could come from alternative R&D models to the private sector model that dominates other therapeutic areas. The challenge with this is that it necessarily restricts the level of funding that can be brought to the field. The top 11 pharma companies spent $104 billion on R&D in 2022, with estimates of total industry spend approaching $250 billion per year (Brown, Amy & Elmhurst, Edwin, 2023; Mikulic, Matej, 2023). At around $50B per annum, the NIH is the world’s most significant funder of early-stage science but we are collectively dependent on industry for the majority of late-stage product investment.
Increased investment in contraceptive R&D by the pharmaceutical industry is unlikely without the potential of robust profits. Many United States citizens have health insurance that covers the cost of the more expensive forms of contraception. The Affordable Care Act requires that private insurers cover FDA-approved contraceptive methods with no out-of-pocket costs for users (Insurance Coverage of Contraceptives, 2022).
Despite the challenges to new contraceptive development, there are also opportunities pointing to a potential promising future. The global contraceptives market had an estimated value of $25 billion in 2019, and is expected to reach at least $38 billion by 2025 (Envision Intelligence, 2022; Speidel et al., 2020). This projected growth is attributed to the widespread efforts of governments, private and public donors, and development organizations to increase access to and use of modern contraceptives and the largest ever cohort of adolescents coming of age (Speidel et al., 2020). While most of the market growth will likely take place outside the United States (Weinberger et al., 2021), this represents a major profit incentive for global contraceptive companies.
A $25 billion market with strong growth expectations represents a market larger than the United States annual drug expenditures for hypertension at $20.5 billion (in 2021), but smaller than the outlays in 2022 for drugs to treat other common conditions including $61.87 billion for diabetes drugs and $121.5 billion for cancer drugs (Aitken, Murray et al., 2022).
As discussed earlier, pharma companies typically spend around 20% of revenue on R&D but for contraception the reinvestment is around 2% (Chamberlain et al., 2020). If big pharma invested the 10% of the $5.5 billion annual United States sales of oral contraceptives their R&D outlays would be $550 million, and 10% of global contraceptive sales of $25 billion would be $2.5 billion a year.
On-demand methods (like condoms) can be purchased without going through the medical care system, but the costs of these methods, while individually low, can accumulate over time (Paying for Contraception in the United States, 2020). There is still a need to support people who would like to access contraception in the United States but face financial barriers.
A manuscript published in 2014 noted that investments in publicly supported family planning save the government $7.09 for each public dollar spent (a net government savings of $13.6 billion in 2010), while helping users avoid not only unintended pregnancy and reducing the need for abortion, but also helping thousands of people avoid cervical cancer, HIV and other STIs, infertility, and reducing preterm and low birthweight births (Frost et al., 2014).
In addition, the contraceptive R&D ecosystem is being strengthened with the support of the public and philanthropic sectors. The NICHD’s Contraceptive Clinical Trials Network, the CTI Exchange, the Male Contraceptive Initiative, and the Initiative for Multipurpose Prevention Technologies are just some of the platforms on which information, news, and resources are shared, collaborations are nurtured, and a new generation of contraceptive researchers are being mentored.
New science needs to be applied to contraception
The past decades have also been a period of remarkable advances in the science and technology needed to develop new pharmaceuticals, including contraceptives (Chamberlain et al., 2020; Committee on New Frontiers in Contraceptive Research et al., 2004). Advances in the areas of genetics and genomics are particularly exciting, allowing researchers to more rapidly and cost-efficiently identify novel targets then identify and validate compounds that work on those targets, and, finally, develop animal models to test them. Artificial intelligence can enable options such as in silico modelling of each of these steps which offers the potential of accelerated R&D timelines.
Efforts are also underway to better understand how individuals will react to specific contraceptive methods using pharmacogenetics and pharmacogenomics – enabling potential users to determine which side effects they are likely to experience (Christofield, 2018; Dama Health, n.d.). Increased funding for and interest in infertility and cancers of the reproductive system may also help contraceptive R&D, especially as the female reproductive system has been historically under-studied. Advances in other fields relevant to contraceptive R&D, including drug delivery, chemistry, engineering, and materials science, also offer potential avenues for acceleration of efforts.
Currently some of the most intriguing new potential drug targets for contraception have been identified through gene expression profiling and related techniques (Johnston & Kopf, 2023). These approaches identify genes only expressed in the reproductive tract. Interestingly males appear to have significantly more of these differentially expressed targets than females suggesting it may be easier to find highly specific male versus female targets. Such advances offer the possibility of discovering novel ways to approach contraception scientifically, beyond the existing hormonal mechanisms which target the hypothalamic-pituitary-gonadal axis. Given hormones tend to have a naturally broad spectrum of effects, and so can give rise to unwanted side effects, the hope is that the new generation of targets will have narrower side effect profiles. There is also optimism that some of the “male” targets can also be used in the vagina to modulate sperm. These might be coupled with other approaches such as pH modulators or ionic spermiostatic agents that alter the vaginal environment to be less hospitable to sperm.
More public and philanthropic investment into the basic science of reproduction and of contraception would also create more starting points from which biotech, pharma and non-profit contraceptive developers could develop contraceptives with novel mechanisms of action. There is recent biotech interest in contraception; as these small biotech companies launch their work on new products, they bring new ideas and energy to the field. Hand-in-hand are the venture capitalists who supply new funding, and the greater risk tolerance needed for early innovations. Most of the reproductive health-focused pharma companies have business models dependent on outsourcing R&D to a thriving biotech segment, because they do not have their own early-stage R&D. Expansion of the biotech and the venture capital community in contraception is thus a critical development. Private sector investments in R&D that are recouped with successful product launches will be key to success in developing the next generation of contraceptives.
4 Clinical Trials.gov: NCT06094283; NCT02927210; NCG03452111; NCT05134428.
5 Data from the G-FINDER online database was analyzed. Selections were made to include development of contraceptives and MPTs, but to exclude microbicides (a subcategory of MPTs not likely to include pregnancy prevention). Not all MPTs in development are targeting a contraceptive indication. G-Finder data does not specify which indication the MPTs are targeting, so this could represent an overcount. However, we also excluded G-Finder categories “R&D for more than one SRH issue” and “Core funding for an SRH organization” as it was not clear if those represented contraceptive R&D or not.
6 Note: a single compound can have multiple clinical trials.
Section VII

Section VII: Summary and Recommendations
Key findings
There is strong demand for contraception, as nearly two-thirds of women+ of reproductive age in the United States use contraception. Tubal ligation, oral contraceptive pills, long-acting reversible contraception, and external (male) condoms are the most commonly used methods (Daniels & Abma, 2020). Yet despite strong usage, we see very high levels of unintended pregnancy - nearly half (45%) of all pregnancies (about 2.5 million annually) are unintended and 42% of unintended pregnancies end in abortion (Guttmacher Institute, 2020b).
While the contraceptive options available on the United States market today appear many, this belies the fact that one category of products, hormonals, dominates with 285 individual branded products almost all based on variants of estrogen and progestin (Drugs.com, 2023), and representing far fewer distinct products. Other than the Copper IUD, all the highly effective non- permanent contraceptives are hormonal.
Pregnancy rates for existing contraceptives entail a considerable risk of having an unintended pregnancy, especially when used under typical conditions. In the first year of typical use of the condom 13 women+ in a hundred, or with the contraceptive pill 7 women+, will fall pregnant. Even perfect use compared to typical use of many methods entails a considerable lifetime risk of pregnancy. For instance, a method with a perfect use annual risk of 0.3% (illustrative of the highest effectiveness products) would translate into about a 7.5% life-time risk of pregnancy if used from 18-45 years old. At an individual level this is a significant risk, at a population level this has highly consequential social and economic consequences.
When somebody is deciding whether and how to contracept, their decision- making is highly personal and influenced by a variety of method-specific attributes people desire in a contraceptive method, such as effectiveness, safety, and having minimal side effects (Coombe et al., 2016; Donnelly et al., 2014; Lessard et al., 2012; Madden et al., 2015; Marshall et al., 2016; Walker et al., 2019). Unfortunately, there are many gaps between the attributes people want and the methods available, forcing some users into trade-offs that may leave them unsatisfied with their method or with no method.
Investment to address these gaps is far below what will be needed to fill them. Without significant increases in funding, we risk waiting many decades to see these important gaps in the method mix filled.
Product attributes desired by users and method gaps
Identification of method gaps facilitates prioritization of product attributes that will better meet people’s needs. Among the most valued attributes that emerged from a review of the literature were effectiveness, safety, minimal side effects (including avoidance of irregular bleeding), ease of use, and duration of effectiveness. The presence of “side benefits” such as amenorrhea, offering protection against HIV/STIs, affordability, ability to be user controlled (requiring less interaction with the health system) and able to be used discreetly, sexual acceptability, and having a rapid return to fertility were also noted as desired attributes. For underserved populations several of the attributes most needed are: having fewer or different side effects and contraindications, offering dual protection from unintended pregnancy and STIs, being able to be used “on demand”, requiring less interaction with the health system, being able to be used discreetly, being affordable, and being for use by men+.
The NewGen Contraceptive Project highlights five major gaps in the current contraceptive option set that could be addressed by new products:
- 1. The lack of highly effective non-hormonal methods: More variety in non-hormonal methods might address hormonal side effect and contraindication profiles and concerns. There is currently only one highly effective and reversible non-hormonal method, the copper IUD.
- The lack of highly effective on-demand methods: There is only one widely used on-demand method, the external condom (Greene Foster, 2020). Nearly a third of reproductive age women have sex less than once per week (Ueda et al., 2020), and thus may prefer not to use a contraceptive method that affects their body every day.
- The lack of highly effective methods that require minimal interaction with the health systems: This is important for anyone living far from care centers and especially for the 19 million women in the United States who are in need of publicly funded contraception and live in “contraceptive deserts (Ueda et al., 2020), as well as for those who are reluctant to interact with healthcare providers, often marginalized populations.
- The need for multipurpose methods: The condom is the only current option that combines contraception with STI and HIV protection. MPTs with high effectiveness rates are especially needed.
- The need for methods for use by men+: Fortunately, there are already groups advocating for and supporting the ongoing development of male methods (e.g., the Male Contraception Initiative’s efforts on non- hormonal male methods).
Affordability also emerges as a priority – so it is critical to understand how novel products that might fill these gaps (which will typically have higher pricing when launched) can be accessed by as many people as possible.
Figure 4 illustrates why these gaps are so critical to fill. It shows the array of current contraceptive choices on the left-hand side, and how they fit with commonly desired user attributes on the right. It is immediately apparent that there is considerable “white space” where no product can address these attributes. We have very few male methods, one STI preventative, and one on-demand product that has proven sufficiently popular for wide usage. What can appear to be a wide range of options, narrows to very few when more than one user attribute is prioritized. Many options fall out when higher levels of effectiveness are required plus an attribute such as on-demand, nonhormonal, self-administered, no prescription, or available from non- specialized providers, such as primary care staff. This “white space” is why we believe there is tremendous opportunity for new product development.
Figure 4: Wide apparent choice but major gaps in the mix
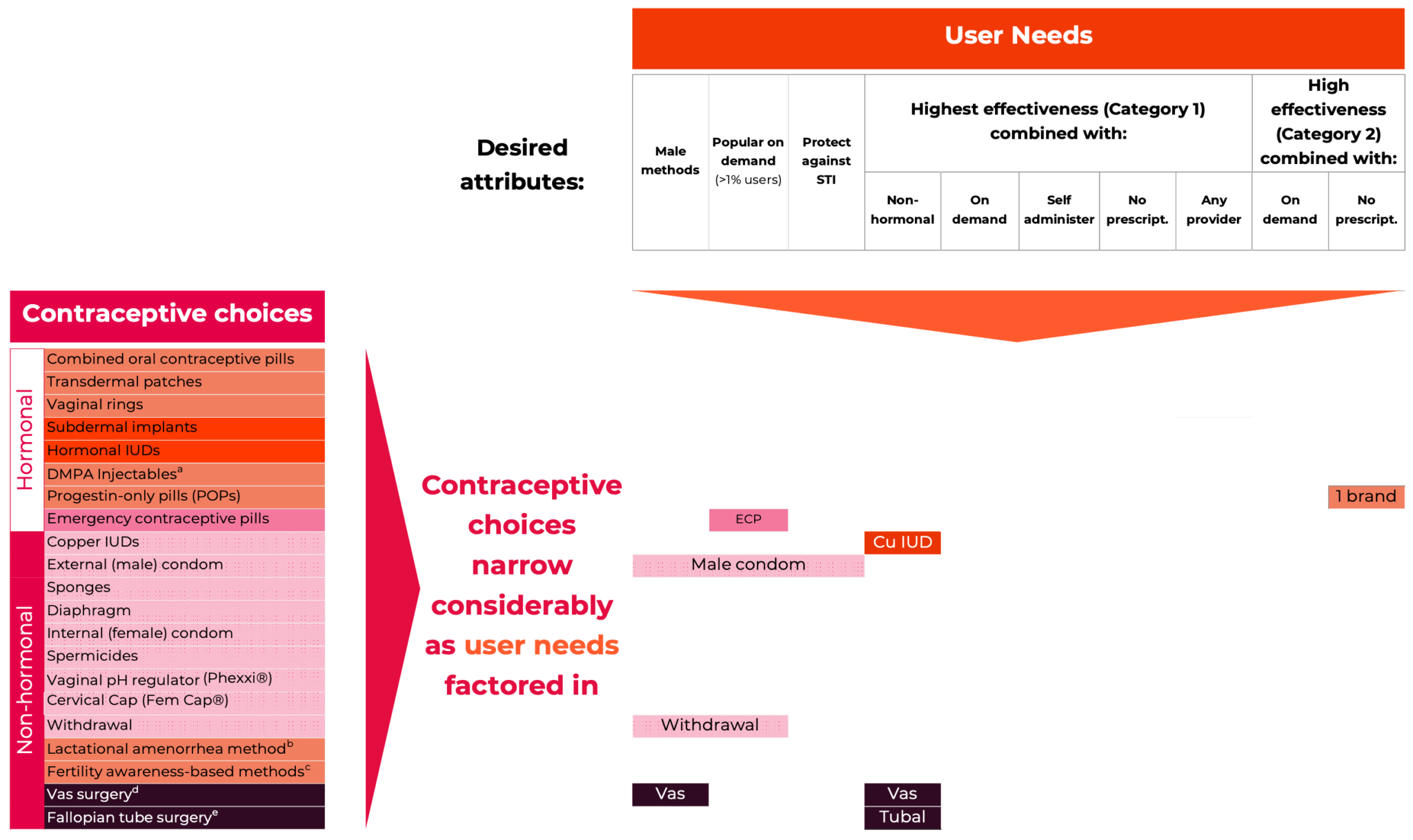
Choices and effectiveness category definitions from Contraceptive Technology 22nd edition (Bradley et al., 2019; Cason et al., 2023). Based on first year of use pregnancy rates Category 1: < 1 pregnancy per 100 women in 1 year with either perfect or typical use. Category 2: 1-7 pregnancies per 100 women in 1 year with typical use. Category 3: More than 8 pregnancies per 100 women in 1 year with typical use.
Data on contraceptive (non)-use, (dis)continuation, and (dis)satisfaction may help with understanding the potential demand for new contraceptive methods. Recent estimates suggest that between 6 and 17% of women at risk of unintended pregnancy in the United States are not using a contraceptive method overall (Fowler et al., 2019; Frederiksen & Ahrens, 2020; Kavanaugh & Pliskin, 2020; Mosher et al., 2015; Pazol et al., 2015), and this proportion may be higher in certain population subgroups.
There is clearly substantial room for improvement in contraceptive satisfaction in the United States population. Quantitative studies from 2000 or later assessing multiple methods available in the general population found that 44-71% of contraceptive users reported being “very satisfied” with their method (depending to some extent on whether use was current or discontinued). While another 25-31% report being “somewhat satisfied.” (See Section IV). The figures on contraceptive non-use further indicate substantial unmet contraceptive satisfaction in the United States.
There are many underserved populations. They include people with health concerns, including side effects, contraindications, and STI transmission; people with needs related to behaviors, such as infrequent sex, a preference for less interaction with the health system, and a need for discrete use; and demographic and socioeconomic groups. These groupings are extensive, they include men+, Black, Hispanic, and other people of color, people who identify as LGBTQ* or gender expansive, those with limited resources or concerns about cost, people with geographic access barriers, younger people, and those older than 40 years. New contraceptive methods are needed to meet the needs of these underserved populations.
While there is growing interest in contraceptive R&D and several recent calls for a contraceptive revolution, the pipeline of truly novel products for women+ in development is limited. Several factors create roadblocks to this work, but the lack of funding is the greatest challenge. Much of the current funding is also heavily weighted towards modifications to existing products (e.g., a smaller copper IUD or new combinations of estrogens and progestins), rather than truly novel products, such as those with new classes of active ingredients.
As abortion access is constrained, these failure rates have increasingly profound impacts (Cason et al., 2023; Guttmacher Institute, 2020b). We need to recognize that our current method mix implicitly relies on abortion to address unintended pregnancy. We need innovation to address gaps in the method mix, including more “set and forget” options and higher efficacy on- demand methods. Alternatives, such as closing the gap between typical and perfect use, have eluded us for decades. We can’t continue to expect that we will “fix the user” through encouraging more perfect use or selection of only the higher effectiveness methods. This would still leave us with high lifetime risks of unintended pregnancy at the population level and we are unlikely to be successful given the trade-offs required by the current option set. We need to “fix the product” with improved contraceptive technology.
Having a greater variety of methods with different attributes increases the chances a contraceptive user will find a method they consider acceptable and can use consistently and correctly. History has shown that with each new contraceptive method introduced into a market, contraceptive use will increase (Ross & Stover, 2013). As such, developing a more diverse variety of contraceptive options would make an important contribution to meeting contraceptive needs and preventing unintended pregnancy.
Changes are needed in the R&D ecosystem
Increase focus on new classes of contraceptives with novel mechanisms of action: New tools and knowledge are available to apply to contraceptive R&D, but the historical under-funding of health research for women+ means product developers are working from a deficit of knowledge about reproductive physiology. The field needs increased investment in the basic biology of human reproduction to identify and validate new drug targets for innovators to pursue. These types of advances are slow, necessarily failure- prone, and hard to predict. Such investments would impact not just contraception but related women’s health conditions which currently face a funding gender gap (Innovation Equity Forum, 2023; Smith, 2023).
The field will also need continued innovation on the existing hormonal and intrauterine methods. While novel contraceptives are needed to address most of the gaps described above, adapting existing methods can provide nearer term improvements to the method mix.
Deepen the bench of contraceptive experts: There already is a remarkable field of contraceptive experts who have been working against the odds given the paucity of funding. But to expand the product pipeline, the field will need to build out its bench of contraceptive experts - and grow the pipeline of people who will be tomorrow’s experts. Attracting more human capital to the field will be essential to success. The field needs research and clinical scientists, engineers and materials science experts, clinicians, statisticians, and experts in regulatory processes, manufacturing, and commercialization.
Build out the biotech and venture community in contraception: The R&D ecosystem will also require greater interest from biotech and venture capital groups because so few large pharmaceutical companies are now conducting in-house research in contraception. In recent years there has been a welcome uptick from biotech and venture capital but this needs to grow and be sustained. Smaller companies can build early-stage pipelines but many will need larger players as partners to bring products to market. So while large pharma in-house R&D on contraception has declined in recent years, it will be important for these companies to invest in the new generation of contraceptives as opportunities emerge from smaller companies.
Address market failures in contraception: The public and philanthropic funders are needed both to sustain – and grow – their investments and support but also to address what appears to be the market failure in contraception. In part through de-risking R&D, but also by ensuring conditions (such as a positive reimbursement, pricing and access environment) reward significant innovation.
For more on market failure in contraception see our commentary in Contraception (Cairns-Smith et al., 2024)
Increased investment is needed to support new contraceptive development
While there is growing interest and some increases in investment in contraceptive R&D, overall investment in contraceptive R&D is not where it needs to be to sustain the existing pipeline of methods. The median cost of bringing a single new drug to market, accounting for failures, was recently estimated at $1.1 billion (Wouters et al., 2020) but estimates commonly range to $2 billion+. With overall annual investments in contraceptive R&D at $149 million in 2022, the field is lacking what is needed to generate a sufficiently strong pipeline to ensure even a few truly novel products get to the market (Policy Cures Research, 2024).
Given the need for greater early-stage science, additional financial support from NIH is warranted. In 2019, the Sexual and Reproductive Health, Rights, and Justice Blueprint called for the NIH and USAID to increase their annual budgets for contraceptive R&D by $122 million (Reproductive Blueprint, 2019). This would have been more than double the United States government’s total spend of $51 million that year (Getting behind Human-Centered Design for Contraceptive Innovation, 2018; Policy Cures Research, 2024). Such increases in the budgets for contraception are critical to advance the field. At the same time, other governments need to step up their commitments as well. And building from the example set by the Bill & Melinda Gates Foundation, we hope to see other foundations and philanthropists enter the field and increase existing commitments. A diversity of funders and actors is important to sustaining a strong and diverse pipeline.
While increased public and philanthropic investment is important, a new generation of contraceptive options will face inevitable capital constraints if we expect philanthropy and the public sector to fill the rapidly declining investment from the private sector. Much greater investment from the private sector is needed to translate new ideas into a robust early-stage pipeline and fund later stage clinical trials, registration and scale-up of manufacturing. But increased private investment, especially from large pharma players, will not materialize without sufficiently attractive markets with rewards for innovation. High-income country markets are typically where the high costs of R&D are recouped, so players need to be convinced that demand is strong for new contraceptives in those markets and that new products will be reimbursed sufficiently to reward investment. Without more high-income market interest and funding of new products, the expansion of contraceptive options will be limited. We are hopeful that growing interest in the field by new entrants, particularly biotech and venture capital, will be sustained and actualized over the coming decades, to create breakthroughs that meaningfully expand the number and diversity of contraceptive methods available to those who need them most.
An increase in overall spending for contraceptive R&D from the current $149 million a year to $500 million to $1 billion7 would be appropriate to move the field forward. The call for this level of investment recognizes that we have multiple product gaps to fill and that the cost of new medical product development is very high.
At a macro level, such increased investment could have significant social and financial returns. Investments in publicly supported family planning save the United States government more than $7 for each public dollar spent (Frost et al., 2014). By helping people avoid unintended pregnancy, these investments will provide important health, social, and economic benefits.
Additional topics recommended for future research
In compiling this paper, NewGen Contraception Project identified several areas where additional research - and programmatic effort - are needed, to support new contraceptive R&D. Much of this work could inform R&D efforts.
Better understanding contraceptive satisfaction and demand for new methods. Although limited, data indicates that substantial proportion of users, and non-users, of contraception are unsatisfied with the contraceptive options and would embrace new methods. A more robust examination of contraceptive satisfaction may provide better, and more current, information on the potential demand both for a particular contraceptive product and for new options in general.
More comprehensive measures of “unmet contraceptive satisfaction,” with key factors and their prevalence in the population and subpopulations, are needed. The factors would span from medical factors such as contraindications and side-effects through attribute preferences whether functional, behavioral or emotional. Such new measures would inform contraceptive R&D developers, funders, and investors of the potential market demand for new methods.
Addressing side effects of hormonal and other contraceptives: A clearer understanding of the magnitude of risk of specific side effects (e.g., weight gain and depression), from hormonal and other methods would be an important input to market sizing efforts, guide goals for research and suggest better ways to educate and counsel contraceptive acceptors. Improved counseling may mitigate some of the impact of side effects by helping people have more clarity about the potential side effects while selecting their method, and by helping people anticipate, understand and manage side effects in a way that feels acceptable to them.
Focusing on (potentially) underserved populations: There are knowledge gaps related to populations potentially underserved by the current contraceptive method mix. For example, individuals at or near peri- menopause those in larger bodies and those with disabilities have been neglected in past research on contraceptive use (Godfrey et al., 2016; Nguyen et al., 2018; Pazol et al., 2015).
Designing new methods that appeal to potential users and deliver high effectiveness under typical use conditions: New contraceptives need to be designed not only to be safe and effective, but also to exhibit other prioritized attributes. Contraceptive researchers have begun using new approaches, including human-centered design, to brainstorm new contraceptive designs with desired attributes (Lawton & George, 2018), for example as a positive part of the sexual experience (Higgins & Smith, 2016; Zaneva et al., 2022). One attribute that requires particular attention given reduced access to abortion is high efficacy and narrowing the gap between effectiveness under perfect and typical use. Accelerating rates of STIs in the United States(CDC, 2021; National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (U.S.). Division of STD Prevention, 2019) and the financial burden of STIs (CDC, 2022) on the United States health system also underline the importance of investing in multipurpose prevention.
Maximizing programmatic efforts to support use of existing contraceptives:
While the existing method mix has gaps that need to be filled, we do have products that have proven safe and effective over many decades of study. Developing new contraceptives is a long-term endeavor. In the meantime, efforts to address contraceptive needs through programmatic means must be undertaken by strengthening family planning information and services. Where specific programmatic changes can successfully address factors limiting contraceptive use, policies need to be changed, programs funded, providers trained, and programs scaled. In addition, operational and behavioral research is needed to identify promising and proven practices that can address contraceptive needs. For instance, there may be ways to counteract disinformation and increase accurate knowledge of contraceptive effectiveness outside of counseling. Contraceptive counseling might be improved to ensure an ideal match for people selecting a contraceptive method.
Conclusion
There is tremendous scope for innovation in contraceptive options. We are optimistic that there will be substantial progress in the coming decades, just as there was a huge burst of innovation in the 1950s, 60s and 70s in response to the dire need at that time. The field has built on those options since, profoundly improving the contraceptive experience. But women+ and their partners still face very high unintended pregnancy rates, even in high income countries with strong access to contraception. With access to abortion constricted in many countries (and increasingly so in the United States), the imperative for more contraceptive options is high.
It is time for a new generation of contraceptive options. A generation built from the extraordinary progress medical science and technology have made in the years since the first hormonal products emerged. A new generation of options could fill important gaps in the current mix and offer products women+ are eager to use, not just tolerated.
But it will require greater financial investment, more human capital, increased risk tolerance, and vision. Few investments promise greater payoffs in individual and family quality of life, and in social and economic welfare.
7 This figure is given to illustrate the order-of-magnitude increase in funding that will be needed to generate a healthy pipeline of new contraceptive options given the $1-2 billion cost of each new drug and the significant outlays for novel devices.
Appendices

The origin of the white paper was our desire to better understand the apparent need for more contraceptive options but the lack of investment to fill those needs. The existing level of investment in the next generation of contraceptives, particularly for novel female methods, is low and risks having little impact on the status quo. Our concern is that we could easily see our children and grandchildren facing a similar set of options, with similar effectiveness, side effect and contraindication profiles, resulting in similarly high rates of unintended pregnancy. In locations where abortion is increasingly restricted this has especially severe consequences.
Appendix A: Why NewGen created this paper?
NewGen Contraception Project’s focus – three areas
There are several possible strategies for reducing the rate of unintended pregnancies and improving the user-experience with contraceptives. A white paper looking at unintended pregnancy and user experience with contraceptives could have focused on the educational, counseling, or behavioral elements of contraceptive usage. It could also have examined in detail contraceptive access and affordability. While we touch on these issues, we do not review them in detail, and we do not attempt to address how health systems could be improved to narrow gaps. These are important issues which we feel many organizations are already working on. It should be noted that high-income countries still face substantial levels of unintended pregnancy, at more than a third of all pregnancies (Guttmacher Institute, 2020a). Given the diversity of healthcare systems and populations in those countries, and the relative ease of accessing contraceptives compared with lower income countries these high levels of unintended pregnancy suggest “fixing the product” is an important angle.
Our focus is on where new contraceptive products could better meet the needs and preferences of users and thereby bring the rate of unintended pregnancies down. This focus comes from the observation that the array of contraceptive options is aging. Most of the current contraceptives have their scientific origin in the 50s, 60s and 70s with innovation since then largely focused on improving on earlier breakthroughs. New products have the potential to better meet users’ needs and preferences.
Given the existing efforts in male contraception, we are optimistic a novel male option will be approved within a decade. But even with new male methods, women+ are likely to continue to carry a disproportionate contraceptive burden, and while there are promising novel approaches in the female contraceptive pipeline, it is still dominated by improvements to existing methods. While these improvements are important and needed, the NewGen Contraception Project believes that a greater number and diversity of products – including more novel products – are needed to fully meet the needs and preferences of women+. As a result, our focus is on novel products for women+, including products with new mechanisms of action and products that combine desired attributes in new ways to offer greater choice.
We focus on expanding investment in contraceptive R&D – especially by re- igniting high-income market interest and investment. In contrast to most therapeutic areas, public and philanthropic players, especially those focused on low- and middle-income countries, have shouldered a substantial proportion of investment in contraceptive R&D (Barot, 2013a; Thompson & Speidel, 2014). This is highly unusual given high-income markets typically invest disproportionately more in R&D and hence drive high levels of product innovation. We think contraception needs (and would reward) this investment, especially to develop products with novel mechanisms of action. The relative underspending shows a lack of recognition of both the need for innovation and the market opportunity in countries with a high capacity to pay for innovation. As a result, in the white paper we focus on the US, a high- income market where consumer demand coupled with a history of rewarding innovation, could foster expansion of investment in contraceptive R&D. However, NewGen Contraception Project strongly believes that increased investment from actors focused on high-income markets would bring resources to the table that would benefit people globally. Further, the needs of all populations should shape investment priorities, as it is critically important to accelerate access to the next generation of contraceptives for all. NewGen Contraception Project would like to see more diversified investment in contraceptive R&D, and we support efforts to address hurdles to contraceptive innovation.
In summary, the white paper reflects three focus areas:
- Focus 1: Gaps that could be addressed by new products,
- Focus 2: Products for women+, with high priority on those with new
mechanisms of action and those that combine desired attributes in
new ways to offer greater choice. - Focus 3: The need for more interest and investment in contraceptive R&D – especially from high-income markets, to complement strong investment by players focused on low- and middle-income markets.
Appendix B: Overview of contraceptive methods used in the United States
Figure A1 – Overview of contraceptive pregnancy rates/usage1
Adapted from Contraceptive Technology, 22nd Edition

1 See Figure 1 main document for explanatory notes and detailed sources. (Cason et al., 2023)
Hormonal Methods
Hormonal contraceptive methods offer both high levels of efficacy and the possibility of other non-contraceptive benefits, like regulation of menstrual cycles, reduced cramping, reduced acne, and amenorrhea (a benefit for some, a negative for others). Some of these methods are also easy to use and do not require action during moments of sexual intimacy. Correctly using different hormonal methods necessitates a range of required actions on the part of the user: Ranging from options taken daily to those providing several months, or years, of protection from unintended pregnancy. Hormonal methods are also associated with various real and perceived side effects, as well as potential health concerns and health benefits, these are described in greater detail below.
The hormonal methods dominate the contraceptive market. In the U.S., the latest count was 285 individually marketed products (Drugs.com, 2023) with the vast majority relatively minor variations of each other. But it is important to note that the vast majority are variants on the same few ingredients in different dosage and delivery forms. Different dosage and delivery forms, and different estrogens and progestins, can be very important innovations for users – but it is important to recognize the limited number of different active ingredients underlying the 285 marketed products in this category.
Combined Hormonal Contraceptives
Combined hormonal contraceptives (CHCs), which contain both estrogen and progestin, suppress ovulation and thicken cervical mucus, thereby reducing the chances of fertilization. The inclusion of estrogen in CHCs results in a more consistent and regular bleeding pattern as compared to progestin- only hormonal contraceptives. Several CHCs exist, including: (1) combined oral contraceptives (COCs) in pill form, (2) transdermal contraceptive patches worn on the skin, and (3) intravaginal contraceptive rings. Each of these options is user-controlled and does not require assistance from a provider to discontinue the method.
In general, CHCs are used consecutively for 21-24 days, followed by 4-7 hormone-free days, during which time a user experiences withdrawal bleeding (Centers for Disease Control and Prevention, 2019). COCs are generally taken each day (with 21-24 days of active pills followed by 4-7 days of placebo pills), patches are generally applied each week (for 3 consecutive weeks, followed by one patch-free week), and rings are generally inserted into the vagina for 21 days, followed by 7 ring-free days. If suppression of menstrual bleeding is desired, these methods can be used in continuous or extended regimens (i.e., not having any hormone-free days or having less frequent hormone-free days).
While the absolute risk8 of major health issues with CHCs are low, CHCs can increase the relative risk9 of certain health conditions. For example, CHC users are at a greater risk of venous and arterial thromboembolism compared to non-users of CHCs, though the existence and magnitude of this increased risk varies by formulation and dose. Notably, these risks are also elevated during pregnancy and post-partum periods (Tepper et al., 2017). These risks also rise with age, so older women are more likely to have contraindications (Allen et al., 2013). CHCs may also be associated with rare but serious health complications, such as cholestatic jaundice or hepatic adenomas. COCs, in particular, are also associated with hyperlipidemia and with a small potential increase in breast cancer risk, while ring use may be associated with rare cases of toxic shock syndrome (although causation is unclear). While certain conditions, such as infertility or birth defects, are perceived by some to be associated with COCs, this is not supported by scientific evidence.
In terms of side effects, some COC users report experiencing unscheduled menstrual bleeding during method initiation or amenorrhea during a time of a scheduled withdrawal bleed, changes in weight, skin, or libido, headaches, mood changes or depression, breast tenderness, or gastrointestinal problems, although not all of these symptoms have been shown to be causally related to COC use. Patch users may experience skin irritation, breast tenderness or soreness, headache, and nausea, while ring users may report headache, vaginal symptoms, irregular bleeding, breast tenderness, nausea, and changes in sex drive. The contraceptive ring, which delivers hormones vaginally, may reduce side effects associated with COCs and is less likely to produce irregular menstrual bleeding.
CHCs offer non-contraceptive benefits, including greater menstrual cycling control. Additionally, COCs reduce bleeding volumes, symptoms of endometriosis, acne, hirsutism, migraines, ovarian cysts, and benign breast conditions. The reduction in bleeding can also mean a decrease in the amount of cramping with menstruation. Because ovulation is suppressed, COC use is associated with a decrease in pre-menstrual symptoms, ovarian cysts, and risk of ovarian cancer. Although there are not data on the long- term health benefits of patches and rings, they are thought to provide similar benefits to COCs.
Progestin-Only Contraceptives
Progestin-only contraceptives (POCs) include: (1) progestin-only pills (POPs), (2) injectables, (3) implants, and (4) hormonal intrauterine devices (IUDs). POPs (often referred to as ‘mini-pills’) are taken orally every day, while injectables are delivered every three months. Implants are thin rods placed by a medical professional under the skin of the user’s upper arm. They are labeled for use for up to three years but are sometimes used for longer durations. Hormonal IUDs require little regular action on the part of the user; they are inserted into the uterus by a health provider and remain effective for 3-7 years (depending on the product). While they are typically removed by a provider, self-removal is possible (with varied success) if necessary.
POCs prevent pregnancy by suppressing ovulation (although norethindrone suppresses ovulation in only a subset of users), thickening cervical mucus, reducing movement of the fallopian tubes, and thinning the endometrium.
Overall, POCs have few serious risks associated with their use (A. E. Burke, 2011). Some evidence suggests increased incidence of ovarian cysts and follicles in progestin-only pill users; although these often resolve on their own and users have little to no symptoms. The strong safety profile of POPs is reflected in the fact that in 2023, the FDA approved Opill (norgestrel), the first daily oral contraceptive approved for use in the U.S. without a prescription (Commissioner, 2023). Rare but serious side effects are linked to the provision process of injectables, implants and IUDs. Severe allergic reactions are rare, but can occur with any type of injection, including contraceptive injectables. Mixed evidence demonstrates a heightened risk for VTE among injectable users, although this effect is thought to be relatively small at the population level. Other rare adverse health conditions associated with injectables include temporary, but reversible, bone marrow density loss in long-time injectable users. Implant insertion may create infection at the insertion site, neural damage, and puncturing. With IUDs, insertion can rarely lead to infection and/or pelvic inflammation. Similarly, incorrectly placed devices may perforate the uterine walls. Risk of ectopic pregnancies among IUD users is rare, however they are life threatening for the pregnant person. In the section below on the Cu IUD, we include more on IUD risks, many of which apply to both hormonal and non-hormonal products.
With POPs, users may report headaches, weight changes, nausea, breast tenderness, fatigue, depression, decreased libido, hirsutism and acne. Menstrual cycles can become irregular during the first few months of injectable, implant, and IUD use. Injectable users also commonly report experiencing weight gain with use, although evidence for causality is mixed. Heavy cramping is common with IUD use: with one study among adolescents and women in St. Louis finding pain and cramping were the most common reason for requesting IUD removal, occurring among 28% of hormonal IUD users and 35% of copper IUD users (Grunloh et al., 2013). Expulsion of the LNG- IUD occurs in about 3-6% of users during the first year of use, and is less common thereafter.
Hormonal IUDs can also be used as emergency contraception (and appear to be as effective as copper IUDs for this purpose) (D. Turok et al., 2022). Non- contraceptive benefits include reduced dysmenorrhea with POPs and implants, and reduced risk of endometrial, and ovarian cancer with IUDs and injectables. Injectables and subdermal implants additionally decrease the risk for ectopic pregnancies.
Emergency Contraceptive Pills (ECPs)
For people who did not use a contraceptive method, or whose method failed during sexual intercourse, ECPs can be used after sex to prevent pregnancy. Three common methods include ulipristal acetate (UPA), levonorgestrel (LNG), and combined estrogen-progestin ECPs used as part of the ‘Yuzpe regimen’ (which involves taking a high dose of regular birth control pills as emergency contraception).
UPA ECPs are the most effective of these three options, but while available over-the-counter in Europe, they are available only as a prescription in the United States. LNG ECPs are approved for over-the-counter use in the United States without a prescription but individual states have different approaches to ECP access (Guttmacher Institute, 2023; KFF Women’s Health team, 2022). Effectiveness of LNG ECPs may be lower in people who weigh more than 165 pounds. The Yuzpe regimen is the least effective of these three options, but if necessary, people with oral contraceptive pills at home can repurpose them for use as EC. ECPs can be taken up to 5 days after unprotected sex, although taking the pills as soon as possible improves their effectiveness. ECPs do not abort pregnancies that have already occurred. After taking the pill, some users experience nausea, or upset stomach.
ECPs are commonly used, and their use has been rising. Analysis by the Kaiser Family Foundation of the National Survey of Family Growth indicated that between 2017 and 2019, 28% of women ages 15 to 44 who had ever had sex with a male reported they had used EC pills at least once in their lives, an increase from 4% in 2002 (Guttmacher Institute, 2016; KFF Women’s Health team, 2022).
Non-Hormonal Methods
Non-hormonal methods vary considerably in effectiveness (Figure A1) but offer contraceptive options for people who wish to avoid the use of hormones given personal preferences or medical contraindications.
Non-hormonal long-acting reversible contraception (copper IUD)
The copper IUD is the only highly effective, reversible, non-hormonal, long- acting, contraceptive option available (Figure A1). It is a T-shaped plastic device with copper wire wound around the stem and sleeves of copper on each arm. The copper IUD primarily prevents pregnancy through two main effects: (1) the presence of a foreign body in the uterus, which makes the intrauterine environment inhospitable to sperm and (2) the effect of copper, which impairs sperm functioning. The device is labeled for 10 years of use but may remain highly effective for longer. In addition, the copper IUD is a highly effective method of emergency contraception. The copper IUD is inserted into the uterus by a provider, and while providers typically also remove it, self- removal by the user is possible (with varied success). This highly safe and effective option is discreet, requires no regular action on the part of the user, and offers a long duration of protection and rapid return to fertility post- removal.
Rare but serious health risks of the copper IUD include pelvic infection, uterine perforation, and pregnancy complications if the method fails. The most common side effect is changes in the menstrual cycle, including spotting, prolonged bleeding, and heavier flow. Many users report this side effect decreases over time, although it is the most common reason for discontinuing this method. More intense or frequent cramping is also common, particularly at the time of IUD placement. A rare, but benign, side effect is IUD expulsion, which occurs in about 3-10% of copper IUD users during the first year of use, and is less common thereafter. Non-contraceptive benefits of copper IUD use include the potential reduction of risk for cervical and endometrial cancer.
On-demand methods
On-demand, or coitally dependent, methods have few medical contraindications. They may be appealing for people who have sexual intercourse infrequently but tend to require more user action for correct and consistent use, leading to lower typical-use effectiveness compared to hormonal or long-acting and permanent methods (Figure A1). Several on- demand methods are female-controlled, which may offer some women enhanced autonomy. Spermicides, internal (“female”) and external (“male”) condoms, and sponges are available over-the-counter, while pH-altering vaginal gels, diaphragms, and cervical caps require a prescription.
Many on-demand methods (pH altering gels, spermicides, internal condoms, diaphragms, sponges, and cervical caps) are inserted into the vagina before sexual intercourse. pH-altering gels are delivered via an applicator, which the user inserts into the vagina, like a tampon. pH-altering gels are effective for up to one hour. A new applicator must be inserted each time the user has sex. pH-altering vaginal gels prevent pregnancy by preventing sperm functioning by lowering the pH level of the vagina (Phexxi®, 2023). Spermicides (including foams, gels, creams, and suppositories) are inserted into the vagina less than one hour before sexual intercourse and can be used as standalone contraception. All spermicides contain chemicals which immobilize sperm and prevent fertilization. External condoms are placed over the penis and internal condoms are inserted into the vagina before sexual intercourse (although these two methods should never be used together). Diaphragms are cup-shaped devices made of silicone that are placed over the cervix and used along with spermicide. If users decide to have sex more than once, more spermicides must be used each time. Sponges are a round piece of polyurethane containing spermicide and with an attached loop to enable removal. Sponges are protective for up to 24 hours and must be left in for at least 6 hours after having sex. Cervical caps are made of silicone and are shaped like a plug with a hollowed-out space that spermicide is placed in before insertion. Relative to the male-condom, none of these methods has so far had strong market uptake.
One health risk of some on-demand methods is the potential for toxic shock syndrome; this has been reported for diaphragms and contraceptive sponges but may be possible for other vaginal methods. Other than slight irritation or the potential for allergy (e.g., latex), most on-demand methods have no associated side effects and few if any contraindications. A key non- contraceptive benefit of some on-demand methods (particularly internal and external condoms), is that they offer protection against sexually transmitted infections (STIs) including HIV. Several on-demand methods can be used alongside other methods to offer dual protection against pregnancy and STIs or to increase contraceptive effectiveness (e.g., external condom with spermicide, etc.).
Other non-hormonal methods
Other non-hormonal methods include fertility awareness-based methods (FABMs), Lactational Amenorrhea Method (LAM), and withdrawal (coitus interruptus). These methods have no side effects, but for some people, can be difficult to use correctly.
FABM users trying to avoid pregnancy monitor various biomarkers that indicate days in the menstrual cycle when the user is most likely to be fertile, and avoid sex without contraception during this time. While the actual window of fecundability is likely to last approximately 6-9 days (Peragallo Urrutia & Polis, 2019), most FABMs generally identify a span of time at least a few days longer than this as potentially fecund, given the difficulty of predicting when ovulation will occur. Several digital health companies have digital applications, some with biomarker tests, which support FABM practices.
The Lactational Amenorrhea Method (LAM) utilizes the natural infertility that occurs post-partum with breastfeeding to prevent pregnancy. To use LAM effectively, the user must not have experienced their first menstrual bleed after delivering (amenorrhea), must be fully or nearly fully breastfeeding (i.e., no interval of more than 4-6 hours between breastfeeds), and must be less than 6 months postpartum. Although this method provides temporary protection, it is highly effective (Figure A1). It can require diligence since all, or the majority of feedings must be breastfeeding.
Withdrawal involves the penis being removed from the vagina before ejaculation occurs, to prevent sperm from reaching the ovum. Many people have difficulty predicting when they ejaculate or having the control to withdrawal, making the typical use effectiveness of this method low (Figure A1). Additionally, even when withdrawal occurs before ejaculation, a small possibility exists that pre-ejaculate could contain sperm (Killick et al., 2011).
Permanent Methods
Tubal ligation (“female sterilization”) is a surgical procedure where the fallopian tubes are closed or blocked, which prevents fertilization. Vasectomy (“male sterilization”) closes or blocks the vas deferens tubes so that sperm cannot travel through the penis. Users still can ejaculate normally, although the semen will contain no sperm. Sperm counts must be taken after the surgery to assure that the procedure is successful; this process takes around 12 weeks before sperm counts reach zero (Centers for Disease Control and Prevention, 2019). Permanent methods are highly effective options for people not desiring future pregnancies, do not require partner compliance, and are discreet.
Because tubal ligation and vasectomies are surgical procedures, there is risk associated with infection or other anesthetic complications. Although these methods are “permanent,” restoring fertility after these procedures is possible, albeit difficult and not always successful. They also require costs associated with the surgery and recovery. Tubal surgery drastically reduces the likelihood of pregnancy. However, for rare pregnancies that do occur after tubal surgery, they are more likely to be ectopic. With both tubal surgery and vasectomy, users rarely experience chronic pelvic or scrotal pain.
Commentary on effectiveness and choice
Perfect-use pregnancy rates convey the proportion of method-users using the method consistently and correctly who are expected to experience a contraceptive failure within the first year of contraceptive use. Typical-use pregnancy rates convey the proportion of all users of a given contraceptive method, including those who use it inconsistently and/or incorrectly, who are expected to experience a contraceptive failure within the first year of contraceptive use. Inconsistent use is common as indicated by a study that noted nearly half of unintended pregnancies occurred in a month when contraception was used (Finer & Henshaw, 2006). As we will discuss in Section VII, these pregnancy rates amount to significant life-time risks of unintended pregnancy. Methods that require more substantial user action to use perfectly, tend to have a larger gap between perfect and typical pregnancy rates. Permanent male and female methods, implants, and IUDs require very little user effort, and are the most effective contraceptives under both perfect-use and typical-use conditions. Other hormonal methods (injectables, rings, pills, and patches) can be extremely effective if used perfectly but require more action on the part of the user – ranging from taking a pill every day to getting an injection every three months – so typical use failure rates are higher. Other methods (barrier methods, FABMs, withdrawal) tend to have the highest perfect and typical use pregnancy rates (Cason et al., 2023). For people who want to avoid a pregnancy, there are only two reversible options that are highly effective, with typical-use pregnancy rates of less than 1%: IUDs and implants. Individuals looking for a highly effective, non- hormonal, reversible, long-acting method have only one option, the copper IUD. Figure A2 illustrates choices ranked by effectiveness including typical and perfect use.
Figure A2: Contraceptive failure comparison
From Guttmacher Institute
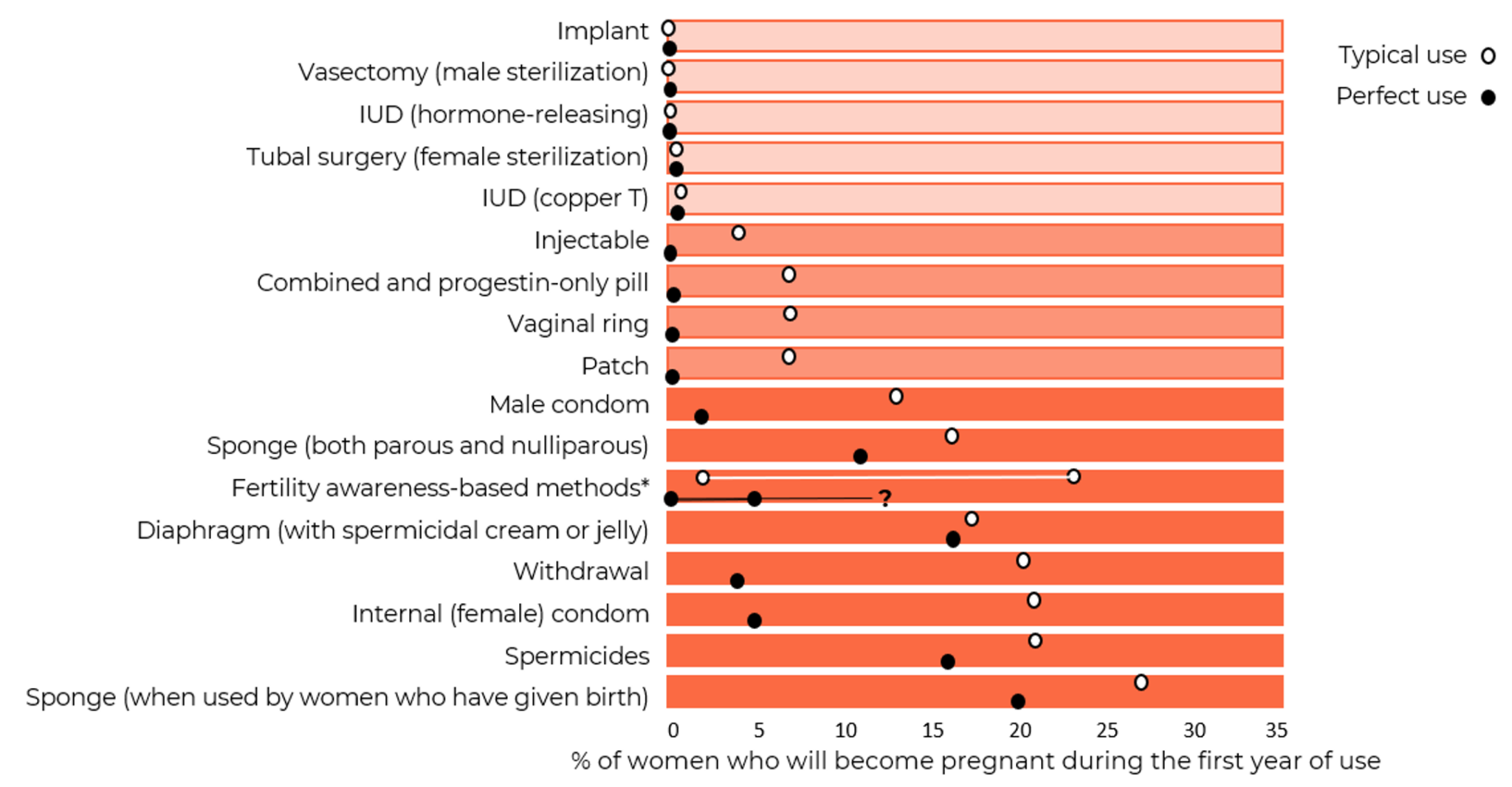
*Range of estimates for FABMs comes from a small number of moderate-quality studies and may not apply to all populations, high quality data are needed (Peragallo Urrutia et al., 2018)
All figures updated from Contraceptive Technology Edition 22nd 2023 (Bradley et al., 2023).
Notes: Typical-use failure rates express effectiveness among all women who use the method, including those who use it inconsistently and incorrectly. Perfect-use failure rates express effectiveness among only those women who use the method both consistently and correctly. Figure by the Guttmacher Institute, available here: https://www.guttmacher.org/sites/default/files/factsheet/contraceptive-effectiveness-united-states.pdf
In interactive chart here: https://interactives.guttmacher.org/contraceptive-effectiveness-31156/.
Appendix C: Contraceptive use and payment patterns in the U.S.
A majority of women (65.3%) aged 15-49 in the U.S. were currently using contraception in 2017-2019 (Figure A3) (Daniels & Abma, 2020). Among all women (including both contraceptive users and non-users), 18.1% reported using female sterilization, 14% reported using oral contraceptive pills, 10.4% reported using long-acting reversible contraceptive methods (including 8.4% reporting use of IUDs and 2% reporting use of implants), and 8.4% reported relying on external (male) condoms. About 5.6% of women reported relying on male sterilization of a partner for their contraceptive method. Only 3.1% of all women reported using injectables, contraceptive rings, or patches, and 5.7% of women reported using all other methods (diaphragm, FABMs, withdrawal, and others). Notably, women who report currently using more than one contraceptive method are classified according to what is considered to be the most effective method (according to a pre-determined hierarchical list) that they report using; studies have shown that this has implications for prevalence estimates of certain contraceptive methods (Polis & Jones, 2018).
Use of contraception increases linearly by age, with current contraceptive use reported by 38.7% of women 15-19, 60.9% of women 20-29, 72.3% of women 30-39, and 74.8% of women 40-49. More non-Hispanic White women report current contraceptive use (69.2%) compared to non-Hispanic Black (61.4%) and Hispanic (60.5%) women. Current contraceptive use overall does not vary significantly according to level of education.
Figure A3: Current contraceptive status of U.S. women aged 15-49
From National Survey of Family Growth, 2017-2019

¹Additional categories of nonusers, such as nonsurgical sterility, are shown in the accompanying data table: see source below.
²Other methods grouped in this category are shown in the accompanying data table: see source below.
NOTES: Percentages may not add to 100 due to rounding. Women currently using more than one method are classified according to the most effective method they are using. Access data table for Figure 2 at National Center for Health Statistics, National Survey of Family Growth, 2017-2019: https://www.cdc.gov/nchs/products/databriefs/db388.htm.
Demographic characteristics
Below, we provide basic demographic characteristics for users of the four most common contraceptive methods in the United States: female sterilization, oral contraceptive pills, long-acting reversible contraceptive methods (implants and IUDs), and condoms (Figures A3 and A4) (Daniels & Abma, 2020).
- Female sterilization: Use of female sterilization increased with increasing age (2.9% of women 20-29, 21.2% of women 30-39, and 39.1% of women 40- 49), declined with increasing education (ranging from 12.1% of women with a bachelor’s degree to 39.9% of women with a GED or less), and was not associated with race/ethnicity.
- Oral Contraceptives: Use of oral contraceptive pills (including both POPs and COCs), is largely concentrated among younger women (21.6% of women aged 20-29 and 19.5% of women aged 15-19 use the pill as compared with 6.5% of women aged 40-49 and 10.9% of women aged 30- 39). A larger proportion of non-Hispanic White women report using the pill (17.8%) than non-Hispanic Black (8.1%) or Hispanic women (7.9%). More highly educated women are more likely to report using oral contraceptives (18.1% of women with at least a bachelor’s degree and 5.7% of women with less than a GED).
- Long-acting reversible contraceptives (LARCs): LARC use (including implants, as well as hormonal and non-hormonal IUDs) is similar among women aged 20-29 (13.7%) and 30-39 (12.7%), and lower among those aged 15-19 (5.8%) and 40-49 (6.6%). There are not significant racial/ethnic differences between current LARC users and non-users of LARCs. Women with some college or with a bachelor’s degree are most likely to report using LARCs compared to women with less education (12.5-13.1% compared to 7.9-9.3%, respectively).
- External (male) condoms: External condom use is highest among women aged 20–29 (10.4%), and 30-39 (9.7%) compared to their younger (15-19, 5.1%) or older (40-49, 6.5%) counterparts. Hispanic and non-Hispanic Black women are more likely to report using external condoms (10.5% and 11.0%, respectively), compared to non-Hispanic White women (7.0%). Use of external condoms does not vary significantly by educational attainment level.
Figure A4: Percentage of women using major contraceptive types, by age group
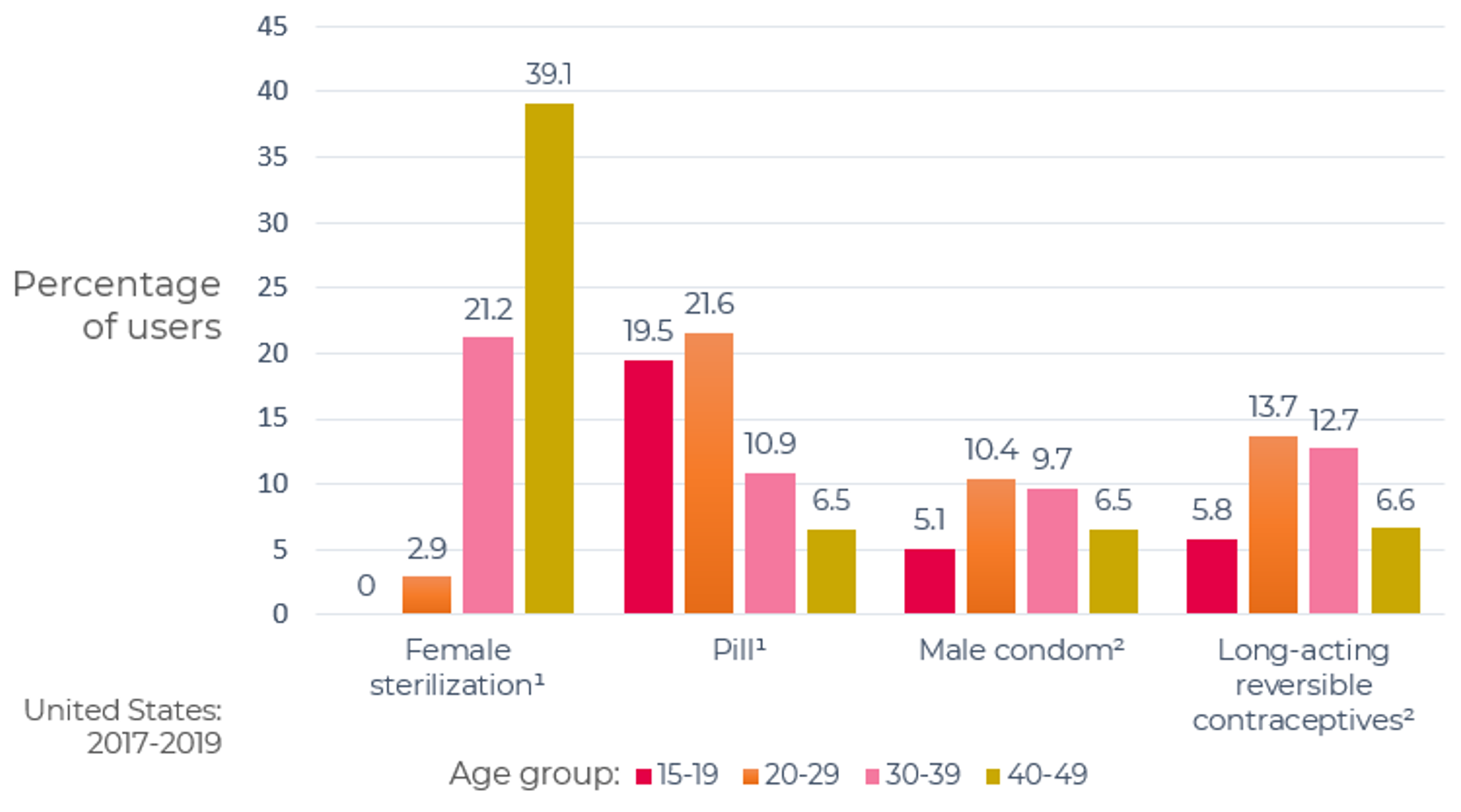
¹Significant linear trend.
²Percentages for age groups 20-29 and 30-39 were higher than percentages for age groups 15-19 and 40-49. Differences for percentages for other age groups were not statistically significant.
Notes: Women currently using more than one method are classified according to the most effective method they are using. Long-acting reversible contraceptives include implants and intrauterine devices.
Access data table at: https://www.cdc.gov/nchs/data/databriefs/db388-tables-508.pdf#3
Source: National Center for Health Statistics, National Survey of Family Growth 2017-2019.
Figure by the National Center for Health Statistics, available here: https://www.cdc.gov/nchs/products/databriefs/db388.htm
Paying for contraception
Financial costs associated with different contraceptive methods vary. For example, insertion of highly effective, long-acting options can cost hundreds of dollars. Since the U.S. has no centralized health care system, people without adequate insurance coverage take on costs associated with these methods. However, requirements of the Affordable Care Act mandate that private insurers cover FDA-approved contraceptive methods with no out-of- pocket costs for users (Insurance Coverage of Contraceptives, 2021). On- demand methods (like condoms) can be purchased without going through the medical care system, but the costs of these methods, while individually low, can cumulate over time (Paying for Contraception in the United States, 2020). There is still a need to support people who would like to access contraception in the U.S. but face financial barriers. More than 20.6 million women in the U.S. are in need of publicly funded contraceptive services (Frost et al., 2019). A recent study using National Survey of Family Growth data demonstrated that among low-income women, 23% would prefer to use another method then their current one and 39% would start using a method if cost was not a barrier (Kavanaugh et al., 2022). A manuscript published in 2014 noted that investments in publicly supported family planning save the government $7.09 for each public dollar spent (a net government savings of $13.6 billion in 2010), while helping users avoid not only unintended pregnancy and reducing the need for abortion, but also helping thousands of people avoid cervical cancer, HIV and other STIs, infertility, and reducing preterm and low birthweight births (Frost et al., 2014).
Appendix D: Contraceptive acceptability: measurement issues
The perspectives of a user on a given contraceptive method is influenced by a wide array of factors, including effectiveness, dosing regimen, perceived and actual side effects, ease of use, mechanism of action, availability, effects on sexual experiences (Higgins & Smith, 2016), method characteristics (size, shape, color, taste, viscosity, etc.), how the method is provided, discreetness, living conditions, attitudes among people close to the user (partners, family members, community members, health care providers, etc.), quality of care and counseling, and more (Mensch et al., 2012). While contraceptive acceptability does not appear to have a singular, agreed upon definition in the field, Elias and Coggins wrote in 2001 that for a method to be acceptable “a potential user must fully understand the potential benefits of using it, the elements of correct use, its potential side effects, and alternative methods and be willing and able to consistently apply such knowledge to the use of the technology in everyday life” (Elias & Coggins, 2001). They suggest that studying contraceptive acceptability is important because it ultimately plays a key role in the method’s typical use effectiveness. Heise noted in 1997 that beyond simply focusing on product attributes, “feminists have argued that ‘acceptability’ must be viewed as a complex interplay between a woman, a technology, and a service delivery environment” (Heise, 1997).
Conceptually, contraceptive acceptability encompasses the hypothetical acceptability of a method prior to use, experiential satisfaction during use, and uptake and sustained method use in the general population (Elias & Coggins, 2001; Minnis et al., 2003; Severy & Newcomer, 2005). Which element of acceptability is studied often depends on where a method is within its development lifecycle, and each has methodological advantages and disadvantages, as briefly described below.
Hypothetical acceptability of a method prior to use: Studies on hypothetical method acceptability aim to provide insight on how potential users might respond to methods in development, which are not yet available for use. One approach assesses the acceptability of specific characteristics of a given method (Delvaux et al., 2021; Hoopes et al., 2018), such as understanding potential users’ concerns about the impact of a vaginal gel on sexual pleasure. Another approach involves asking people to make trade-offs between various features of a hypothetical method (e.g., would potential users accept irregular menstrual bleeding if they didn’t have to remember to take the contraceptive at the same time each day) (Delvaux et al., 2021; Severy & Newcomer, 2005). Other studies attempt to make inferences based on acceptability data from similar methods (e.g., using data on spermicides and sexual lubricants to better understand users’ potential preferences for microbicides in development) (Elias & Coggins, 2001). While these approaches can be useful for obtaining end-user input early in method development, some studies suggest that people’s responses to hypothetical methods often do not align with actual use (A. Glasier, 2002; Minnis et al., 2003; Ravindran et al., 1997).
Experiential satisfaction during use: The concepts of contraceptive acceptability and contraceptive satisfaction are closely linked, but satisfaction is generally considered a dimension of overall acceptability (Delvaux et al., 2021). Satisfaction tends to be assessed by asking users to rate their level of satisfaction during method use, or via indirect methods, like asking about willingness to use the method in the future or to recommend the method to a friend (Delvaux et al., 2021). Many such studies are nested within clinical trials testing the safety and efficacy of contraceptive methods in development (Severy & Newcomer, 2005). This can mean that participants do not have full information about key attributes of the product (safety, effectiveness), making even experiential satisfaction of the method somewhat hypothetical (Woodsong & Alleman, 2008). Generalizing the results of clinical trials to broader populations can be challenging for several reasons. For example, people who agree to participate in clinical trials are often highly selected, and clinical trial participants often receive more intensive clinical care than may be typically received outside of a trial setting (A. Glasier, 2010). For this reason, findings from acceptability studies nested in clinical trials may be useful to method developers, but may not accurately convey how a method will be received in the general population (Elias & Coggins, 2001).
Also, while clinical trials are well-suited to elucidate the frequency of certain side effects, they may be less well-suited to understanding the user’s subjective perceptions of and responses to those side effects (Rominski et al., 2017). Some researchers have argued that questions commonly used to measure experiential satisfaction in clinical trials are sometimes overly simplistic (e.g., a handful of statements for which the participant is asked to rate their level of agreement (A. Glasier, 2010). One study assessing method satisfaction via four questions (whether the participant liked the method; whether she thought her partner liked the method; whether sex with the method was the same, better, or worse; and whether she would recommend the method to a friend) found that – unexpectedly – high satisfaction was generally not correlated with future use of the method, but that low satisfaction was generally correlated with non-use of that method (Minnis et al., 2003).
Another important factor to consider in interpreting results from studies of contraceptive satisfaction is whether the study includes only current users of the method, or includes both current and former users of the method, as the latter group may include those who discontinued the method due to low satisfaction (thus, not including them may artificially inflate apparent satisfaction measures) (Moreau et al., 2007b).
Uptake and sustained use over time in the general population: After a method is approved for marketing, the appeal of that method to the broader population can be assessed – along with how use is impacted by characteristics of the user, the user’s sexual partner(s), characteristics of the product, the health system in which the method and alternative methods are provided, and the user’s socio-cultural context (Elias & Coggins, 2001; Severy & Newcomer, 2005). Studies can occur prior to actual market introduction, such as by showing a method to potential users and inquiring if they would be likely to try it (Elias & Coggins, 2001). Other studies may investigate experiences during actual method use. This may also involve assessing which features of a method are liked or disliked, and inquiring about intentions to use the method in the future (Minnis et al., 2003). One component assessed during studies of longer-term use may involve the propensity to discontinue the method. Indeed, some scholars have conceptualized contraceptive acceptability as “the voluntary sustained use of a method in the context of alternatives” (Severy & Newcomer, 2005). However, as described above, contraceptive discontinuation may not always indicate lack of method acceptability. And conversely, contraceptive continuation may not always reflect method satisfaction – but may sometimes reflect passive continuation of the “least bad” option a person believes to be available (A. Glasier, 2010), or even challenges in access to services for method cessation.
Once a method is publicly available, method prevalence is sometimes considered another indirect measure of method acceptability, as this provides an indication of how widely a method is chosen for use in a given population. However, it is important to bear in mind that method choice is itself influenced by an array of other factors (A. Glasier, 2010), including method accessibility, cost, influences from social networks, providers, and more.
Given the contraceptive method acceptability measurement challenges described, we did not comprehensively review literature on hypothetical product acceptability, nor acceptability data from clinical trials of individual methods (particularly since the latter is often more widely available for newer methods or methods in development (A. Glasier, 2010). Instead, we focus on summarizing key studies on contraceptive (dis)satisfaction in the United States that assessed multiple contraceptive methods (vs. focusing on individual methods), prioritizing nationally representative studies, where possible. We examined quantitative studies, since relevant qualitative studies were often focused on particular populations or methods (e.g., (Gomez et al., 2020; Higgins et al., 2015)). Finally, we report only on data collected since 2000, although earlier studies are available (e.g., (Oddens, 1999; Rosenberg et al., 1998; Rosenfeld, 1993)).
In summary, research that aims to measure contraceptive acceptability, including satisfaction, has faced challenges in conceptual clarity, measurement, and interpretation (Heise, 1997), and how acceptability is measured often depends on whether the product is currently hypothetical, in clinical trials, or available on the market. We summarized quantitative studies from 2000 or later assessing multiple methods available in the general population. Across these studies (the most representative of which occurred nearly 20 years ago), 44-71% of contraceptive users reported being “very satisfied” with their method (depending to some extent on whether use was current or discontinued). While another 25-31% report being “somewhat satisfied,” there is clearly substantial room for improvement in contraceptive satisfaction in the US population. In these studies, certain characteristics were associated with contraceptive satisfaction, including method attributes (e.g., ease of use, perceived effectiveness, side effects, privacy), method accessibility (e.g., cost), individual characteristics (e.g., age, race/ethnicity, partnership status, health status, recency of sex), and counseling experiences (e.g., experiencing shared decision-making, not feeling the provider had a preferred method).
A linked concept to satisfaction is unmet need for contraception. Unmet need for contraception is often conceptualized as pertaining to whether an individual at risk of unintended pregnancy is using a method or not. Is this a high enough standard, particularly as it pertains to interventions used by generally healthy people, by the majority of those at risk of unintended pregnancy, and with use often spanning multiple decades? What would aiming to address “unmet contraceptive satisfaction” look like, and would it be viewed as a worthy goal by contraceptive developers, manufacturers, providers, policymakers, and other stakeholders?
Appendix E: The Current Contraceptive Pipeline
FHI 360 maintains a database called Calliope, the Contraceptive Pipeline Database, (https://pipeline.ctiexchange.org/) with nearly 250 entries covering potential contraceptive targets and leads in early development, products in pre-clinical and clinical development, and a selection of products with limited market availability outside the US. Despite this impressive number of entries, many are no longer actively being developed and many of the products with limited market availability would not be desirable for US market introduction. Further, the low success rates associated with R&D mean that a likely outcome from the current pipeline is no new products with novel active ingredients. Setting aside earlier stage and non-U.S. entries, there remains 87 products in development, of which half (44) are female, hormonal products (mostly modifications to existing products) and almost all of the other female products in clinical trials are variants on existing methods, e.g. different combinations of emergency contraceptives, different IUD designs. There are 6 active male hormonal products in the database. The remaining non- hormonal products are nearly evenly split between female (20) and male (17). Most of these (27) have not yet reached the clinical stages of development (FHI 360, 2023a). A review of the US clinical trials registry (clinicaltrials.gov) revealed only 12 active phase 1-3 clinical trials of contraceptive methods as of December 2022 (Home - ClinicalTrials.Gov, As accessed).
As discussed above, later stage contraceptive product development efforts on the female side are largely focused on modifying existing methods with very few entirely new products. Modifications generally include changes to the dosing or the delivery of the active pharmaceutical ingredient (API), with the aim to extend the duration of effectiveness, improve convenience, or reduce side effects. Delivery system changes may be alterations in the size and shape of the delivery system, such as smaller IUDs, or using smaller, subcutaneous (versus larger, intramuscular) needles, or entirely new delivery systems, such as microneedle patches or fast-dissolving inserts.
There are some more substantial modifications of existing products, for instance through the use of newer progestins designed to improve the safety and/or side effect profile (Haddad, RamaRao, et al., 2021). These changes incur higher regulatory scrutiny but benefit from having a known mechanism of action. Compared to completely new product development, modifications to existing products are lower-risk because the specific API and/or the mechanism of action is already proven and thus lower cost. Modifications can improve upon a product and make it more desirable to users (for instance, by decreasing side effects or being more discreet), but there are limits to what can be changed. To address the biggest gaps in the current contraceptive method mix, including the development of a highly effective, reversible, and non-hormonal option (other than the copper IUD) and substantive improvements to on-demand methods, developers need to find a new generation of APIs with alternative mechanisms of action and new delivery options. This work requires much more substantial investment and greater tolerance for failure. There are also interesting early discovery stage targets but these require considerable resources to conduct IND-enabling studies and human clinical trials.
The following are some examples of promising products in the current pipeline. Products are grouped by the sex of the intended contraceptive user and by hormonal and non-hormonal (Figures A5 and A6). Products in clinical and the later stages of pre-clinical development are described. The description of the female non-hormonal pipeline highlights a few leads that are in early pre-clinical development, targets, and leads in discovery. For the early male contraceptive pipeline, several recent reviews and the Male Contraceptive Initiative’s Non-Hormonal Reversible Male Contraception Pipeline Database (https://www.malecontraceptive.org/nhrmc- database.html) already provide thorough discussion of targets and leads (Long et al., 2021; Male Contraceptive Initiative, n.d.; Thirumalai & Amory, 2021). For context, the products most recently introduced to the US market are described.
Female non-hormonal products: Among the female non-hormonal products in clinical development, perhaps the most exciting is OvaPrene® ((Daré Bioscience, 2023)Daré Bioscience Inc.). OvaPrene is a monthly intravaginal contraceptive, ring-like device which during the course of one menstrual cycle releases an agent that impedes sperm mobility and access to the cervical canal. A phase 3 trial of OvaPrene is planned (Daré Bioscience, 2023). Two variations on the copper IUD have recently completed Phase 3 trials: VeraceptTM (Sebela Pharmaceuticals) (Sebela Women’s Health Inc., 2022), which uses a flexible nitinol frame and a lower dose of copper than the currently marketed product (Paragard®) and the Mona Lisa® NT Cu380 Mini (Mona Lisa, N.V.) (Health Decisions, 2021), which uses a smaller frame than Paragard. Final results from these studies are not yet available. Interim results for the Mona Lisa product, however, indicate improved continuation rates and decreased side effects among nulliparous women (Hubacher et al., 2022). It is similarly hoped VeraCept will also offer improved acceptance, safety, and side effects while maintaining efficacy on par with Paragard (D. K. Turok et al., 2020). Ulipristal acetate has also been added to the copper IUD, demonstrating that it prevented the increased bleeding often experienced by copper IUD users (Brache et al., 2021). “Frameless” IUDs, including the intrauterine ball Ballerine® (Ocon Medical Ltd) and Gynefix® (Soyin), and FibroPlant®, also have the potential for improved performance compared to the standard T-shaped IUDs (Baram et al., 2020; Wildemeersch et al., 2014). While such products are available in some European countries, none have been approved for use or entered clinical testing in the US. Additional frameless IUDs are being developed e.g. 3D-001 from 3Daughters (3Daughters, 2023). Meanwhile, the pivotal trial of what may lead to a new non-surgical approach to permanent female contraception, FemBloc® (Femasys Inc.), has been announced (Femasys Inc., 2023). Other non-surgical permanent – and potentially reversible - contraceptive approaches that block the fallopian tubes are further from potential approval in the US. There is also increasing interest in the development of contraceptives using progesterone receptor modulators, ulipristal acetate and mifepristone. Ulipristal acetate is already marketed as a form of emergency contraception and mifepristone as one of two drugs in the medical abortion regimen. There is interest in both as pericoital or on-demand contraceptive options (Cahill & Blumenthal, 2018; Snodgrass, 2022). An initial study of ulipristal acetate plus meloxicam, an NSAID, showed promising results for this combination to be used as an oral pericoital option (Cahill et al., 2022). A contraceptive trial of a weekly 50mg dose of mifepristone is now underway in Moldova (EU Clinical Trials Register, 2023).
Several new non-hormonal on-demand methods are also in pre-clinical development. Oui (Cirqle) is a vaginal product that reacts with the cervical mucus to make it impenetrable to sperm (Cirqle Biomedical, 2022; Schimpf et al., 2022). Polyphenylene carboxymethylene (PPCM) (Yaso Therapeutics) is a vaginally-delivered product with a novel polymer and potential as an MPT (Weitzel, 2019). Pre-clinical work is underway on combining Phexxi® (EvoFem), a recently approved contraceptive vaginal gel, with a novel compound to create a non-hormonal MPT (Evofem Biosciences, 2022). There is also early work on a naturally occurring antimicrobial peptide, LL-37, to explore its potential as a vaginally delivered MPT (Kiattiburut et al., 2018; Vera- Cruz, 2022).
There are also early stage and clinical efforts to develop contraceptive monoclonal antibodies that target sperm/additional targets in the vaginal canal, potentially as part of an MPT formulation (Anderson et al., 2020; Baldeon-Vaca et al., 2021; Shrestha et al., 2021)). The products would likely be an on-demand film e.g. HCA/ZB-06 film from ZabBio which is entering Phase II clinical trials in 2024 (ZabBio, 2023). Another non-hormonal MPT vaginal ring, designed to prevent unintended pregnancy, bacterial vaginosis, and the transmission of HIV and several other STIs, is in preclinical development. Its active ingredients include copper, zinc, and lactide (Population Council, 2023; Rubens, 2022). Also in development is an on-demand, non-hormonal MPT fast-dissolving vaginal insert aiming for the same indications as the non- hormonal MPT vaginal ring (Derby et al., 2018; Population Council, 2023).
R&D of novel pharmaceutical approaches with new mechanisms of action usually begins with identification and validation of a biological target (usually a gene or a protein), followed by identification and optimization of compound(s) acting on those targets. While there are several targets for male contraception being studied, the research on female targets is much slimmer. The cation channel of sperm (CatSper) is a validated target with several potential compounds in the R&D process (Carlson et al., 2022). While CatSper activation is essential for sperm motility, it is activated in the vaginal canal. Thus, while it is often seen as a potential target for male contraception, it also has the potential to be used vaginally as a female method (Rennhack et al., 2018). Groups, including Daré Bioscience (Daré Bioscience, 2023), are pursuing identification and optimization of compounds that block activation of CatSper for potential use by all genders.
Other potential targets for the development of female contraception include phosphodiesterase (PDE) 3A, Wee2, Juno, (Blithe, 2016) and prostaglandin E2 (Duffy, 2015). PDE 3A and Wee2 inhibitors prevent oocyte maturation and efforts to identify potential leads for development into contraceptives is underway (Hanna et al., 2020; Jakkaraj et al., 2018; Jensen, 2015). Juno, a target related to egg-sperm fusion, is also being studied for potential leads (Bianchi et al., 2014; Stepanenko et al., 2022). Prostaglandin E receptor antagonists would likely work by preventing follicle rupture (Trau et al., 2016), and early animal tests have showed promising results (Peluffo et al., 2014).
Female hormonal products: One approach to expanding the range of hormonal options is to change the delivery system to make use more discreet, require less interaction with the health system, or otherwise be easier and more acceptable. There is a once-a-month progestin-only pill in pre-clinical development that would decrease the need for daily pill adherence while still being user-controlled (Kirtane et al., 2019). Delivering a progestin (and potentially an estrogen and/or HIV or STI prevention drug) via microneedle patches (also known as microarray patches) would provide users with a more discrete option than existing transdermal patches. Several contraceptive microneedle patch approaches are in pre-clinical development, with varying durations of effectiveness (Altuntaş et al., 2022; W. Li et al., 2019; Yavuz et al., 2020). There are clinical trials of 6-month subcutaneous DMPA products, which would decrease the number of provider visits required for the current 3-month product (Halpern et al., 2021; Teva Branded Pharmaceutical Products R&D, Inc., 2022). Other approaches to develop a longer-acting injectable, with progestins other than DMPA, are in pre- clinical development (Daré Bioscience, 2023; MedinCell, 2022). Several groups are exploring approaches to biodegradable implants that do not require removal by a healthcare provider. Progestin-only and MPT approaches are in pre-clinical development (Cohen et al., 2023; FHI 360, 2023b; HeraHealthSolutions, n.d.; L. Li et al., 2021). Finally, a microchip contraceptive has been designed to combine the benefits of long-acting methods with the user control of short-acting methods. The microchip, which is in preclinical development, would deliver hundreds of doses of a progestin over many years, but could also be turned on and off by the user to allow for pregnancy (Daré Bioscience, 2023).
Using alternative APIs is another approach to expanding the range of hormonal options. Most marketed combined hormonal contraceptives use ethinyl estradiol (EE). Alternatives, including synthetic or natural estradiol (E2) and estetrol (E4), have started to reach the US and European markets as part of contraceptive formulations and are potentially safer than EE (Blithe, 2016; Fruzzetti et al., 2021; Haddad, RamaRao, et al., 2021). There is also a great deal of interest in newer progestins for improved safety and side effect profiles (Guttmacher Institute, 2020a; Sitruk-Ware, 2006). Again many of these, such as drospirenone and segesterone acetate (Nestorone®), have begun to reach the market. Finally, an androgen restored contraceptive (Pantarhei Bioscience / Gedeon Richter) aims to decrease some of the side effects and risks associated with COC use by including a natural human androgen dehydroepiandrosterone (DHEA) to normalize testosterone levels during use (in addition to an estrogen and progestin). There is a Phase IIb/III clinical trial ongoing in Europe (Pantarhei, 2023; van Lunsen et al., 2018).
There are several exciting advances in the realm of hormonal MPTs. Within this class, the dual prevention pill (Viatris; Population Council and Medicines360) is potentially the closest to market. This daily oral pill combines an estrogen (ethinyl estrogen), progestin (levonorgestrel), and an antiretroviral (tenofovir disoproxil fumarate; brand name: Truvada®) (Friedland et al., 2021; Population Council, 2021). Because it is a combination of already approved and marketed products, it requires only a bioequivalence study rather than a full Phase 2 clinical trial. Two hormonal MPT vaginal rings are also in clinical development (PrEPWatch, 2022). The first combines levonorgestrel and tenofovir and is in Phase II trials (CONRAD) (Thurman et al., 2022). The second combines levonorgestrel with dapivirine, another antiretroviral, and is in Phase I trials (Population Council) (International Partnership for Microbicides, Inc., 2022). Several other products combining a progestin (and possibly an estrogen) and an antiretroviral, packaged as vaginal rings, injectables, microneedle patches, and implants are in preclinical development (Initiative for Multipurpose Prevention Technology (IMPT), 2022; PrEPWatch, 2022). More information on these products is available from the Initiative for Multipurpose Prevention Technology (IMPT) Product Database (https://mpts101.org/) and the PrEPWatch Research Pipeline (https://www.prepwatch.org/research-pipeline/).
Male non-hormonal methods: In the past decade, attention and scientific effort has focused on non-hormonal contraceptive development. Most of this work is funded by NICHD, and the Male Contraceptive Initiative, a 501(C)3 based in North Carolina, has helped to spur attention to these efforts (www.malecontraceptive.org). The male reproductive process offers several interesting targets and there is a small community of researchers who have been working in this area for the last couple of decades (Vahdat et al., 2020).
The only recent innovation that has reached the clinical stage is ADAMTM (Contraline), which launched its first-in-human study (pre-pilot) in November 2022 (Contraline, Inc, 2022). ADAMTM is a hydrogel that occludes sperm from flowing through the vas deferens for a predefined period of time; the gel eventually degrades so the approach is not permanent. ADAMTM is delivered via a minimally invasive, no-scalpel approach. A similar approach is Vasalgel (Parsemus Foundation, 2023; Plan ATM | Reversible Male Birth Control, 2023), which is in a late preclinical stage. Retinoic acid receptor antagonists and biosynthesis inhibitors have been shown to temporarily block sperm formation. Several show promise in early preclinical work, with one, YCT529, poised to enter Phase I in late 2023 (American Chemical Society, 2022; Chung et al., 2016; YourChoice Therapeutics, 2023).
Other male non-hormonal approaches focus on novel targets. EP055 (Eppin Pharma) is a compound in pre-clinical development that targets the sperm surface protein EPPIN, thereby inhibiting sperm motility. Animal studies indicate that it has the potential to be a short-acting, reversible male contraceptive (O’Rand et al., 2018). Triptonide, a derivative of the Chinese herb Tripterygium Wilfordii, likely targets a process in late spermiogenesis, resulting in malformed and nonmotile sperm. Results from studies in mice and monkeys showed promise for its development as a short-acting, reversible male contraceptive (Chang et al., 2021).
There are many other non-hormonal male technologies that are further back in the pipeline, of which only a few of the furthest along are discussed here. Soluble adenylyl cyclase (sAC) inhibitor affects sperm capacitation and motility and could be packaged as an oral on-demand male contraceptive (Balbach et al., 2020; Rossetti et al., 2021). Ouabain derivatives show promise as inhibitors of the Na+/K+-ATPase plasma membrane pump, which is essential for sperm development and motility (Syeda et al., 2018).
Male hormonal methods: In 2011, the first, large Phase II study of a male hormonal method, an injectable combining testosterone and a progestin, was terminated early due to the high number of reported adverse reactions (i.e., side effects, including mood disorders) (Behre et al., 2016). Another large, Phase IIb study of a different male hormonal method is now underway, this time with a self-administered (daily) gel consisting of testosterone and Nesterone (a progestin). This study, led by the Population Council and NICHD, is currently underway at sites in the US, Africa, Europe, and South America (NICHD, 2022; Thirumalai & Amory, 2021). An alternative male hormonal approach is to use a novel androgen, which has the potential to act as a single agent male contraceptive. These include two potential pill formulations: dimethandrolone undecanoate (DMAU) and 11b-methyl- nortestosterone dodecylcarbonate (11b-MNTDC) (Thirumalai et al., 2019; Yuen et al., 2020). Both have completed initial clinical trials, reported promising results, and are being further evaluated.
Newly approved products: At least six new (non-generic) contraceptive products have been approved by the US FDA in the last 10 years. Four of these are hormonal and two are on-demand methods.
- Annovera®, approved by the FDA in 2018, is a combined hormonal vaginal ring with a one-year duration of effectiveness. It uses a very low dose of estrogen and the novel progestin Nestorone and is removed for a week each month to allow for a period of withdrawal bleeding.
- In 2019, Slynd®, the first progestin-only pill to use the progestin, drospirenone, was approved by the FDA. It is designed to have a longer missed pill window and decreased irregular bleeding than other POPs.
- In 2021, Nextstellis®, the first contraceptive to use the Estetrol (E4), along with a progestin (drospirenone), was approved by the FDA.
- Twirla®, approved in 2020, is a weekly transdermal patch with a lower- dose and alternative progestin to the existing patch products.
- Caya® is a one-size-fits-most diaphragm. It does not require a fitting, but does require a prescription and the use of a spermicide or other contraceptive gel. It has been available in the US since 2015.
- Phexxi®, formerly known as Amphora, is a pericoital contraceptive gel that makes the vaginal pH inhospitable to sperm. Phexxi® was approved by the FDA in 2020.
In addition, two digital fertility tracking apps have been cleared by the FDA to be marketed as contraceptives: Natural Cycles (cleared in 2018) and Clue (cleared in 2021). Natural Cycles requires use of a basal body thermometer, tracking of menstrual cycle start dates, and optional urinary hormone testing. The Clue Birth Control app is based on the Dynamic Optimal Timing Method, but is not currently available in the United States. Companies, like Inne and Eli Health, that provide biomarker analyses such as measurement of salivary hormone levels are also emerging. Their services could be an adjunct to other FABM methods or the basis for new FABMs combined with their biomarker platforms (Eli Health, 2023; Inne, 2023).
Figure A3a: Female contraceptive R&D pipeline
Selected examples

Figure A3b: Male contraceptive R&D pipeline
Selected examples
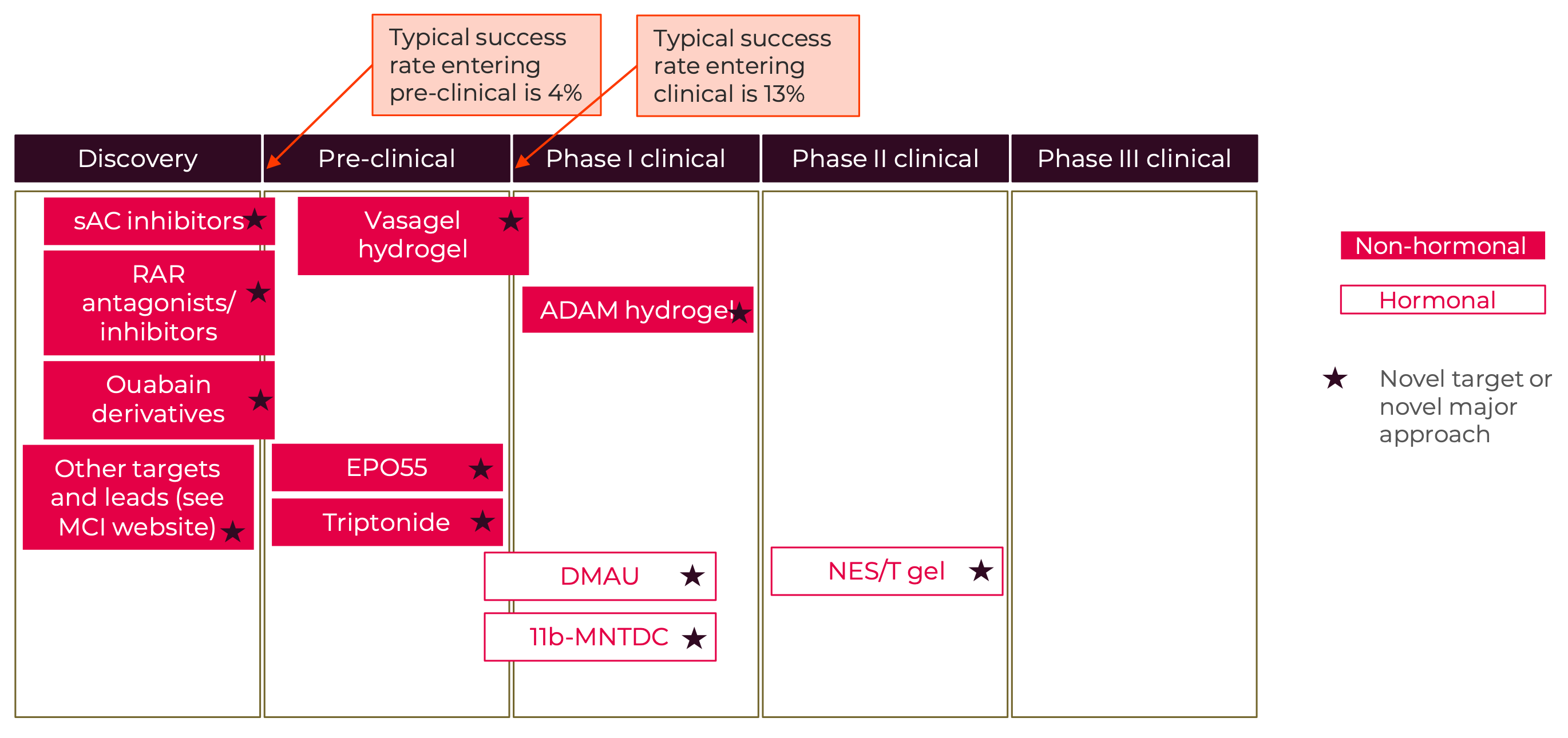
Appendix F: Potential Role of NewGen Contraception Project
NewGen Contraception Project was formed because the founders saw a need to engage women+ more directly in communicating the need for new options and pointing to the lack of investment in this critical area of their health care. Engaging women+ more in the shaping of R&D investments and product design can have tremendous benefits. We would like to see an energetic and collaborative relationship between women+, researchers, innovators, investors, regulators, and payers to get the best new products to market faster - in the US and globally.
In medical research, there is a lot of discussion about “patient centricity,” but it can be hard to figure out how to implement this concept effectively. NewGen Contraception Project wants contraception to be hailed as leading the charge in “smart user centricity” – with women+ and their allies as convenors of working groups on pre-competitive R&D issues, as advocates for greater investment and more investors, as engaged stakeholders of innovators and regulators, and as generators of data for decision-making. We want to help to build a vibrant, informed community of contraceptive users that share in the successes and understand the failures of the field.
While we see ways in which more user-centric approaches can support bringing better products to market faster, without more investment, the range of options will remain limited. As such, the other focus of NewGen Contraception Project’s work will be to bring more total investment to the field. While NewGen Contraception Project would like to directly invest in the research, for instance through catalytic grants for young investigators, the sheer cost of product development means that the major investments will come from other players such as government, major non-profits, foundations, and industry. We will advocate with women+ for increased investment in contraceptive R&D by clarifying the potential market opportunities and improving the understanding of user needs.
NewGen Contraception Project will prioritize communities that are particularly affected by gaps in the current range of products by ensuring their needs get sufficient attention from decision-makers. To do this, we will engage underserved populations on their perspectives, needs, and preferences for future methods.
Central to our approach will be partnership. We will partner with health care providers, contraceptive scientists, innovators, funders, and other decision- makers on priorities for new methods and future research directions, bringing both user advocates and more user-focused data to those partnerships. We will convene stakeholders to discuss the hurdles facing contraceptive developers and explore ways to overcome them. Through all of this work, women+ will be at the center.
NewGen Contraception Project aims to support contraceptive innovation in several ways:
The first is to increase awareness of the need for a new generation of options. The success of the hormonal products has in many ways obscured the gaps in the method mix because the optimization of hormonal methods has generated a consistent pipeline of modifications. We aim to raise public awareness by bringing more attention to the major gaps in the options and the needs of women+.
Second, New Gen will support calls for increased funding of research and innovation and raise private, philanthropic and public interest in new contraceptive R&D. NewGen will work both within the contraceptive R&D ecosystem to advocate for increased funding and elevate public pressure and consumer advocacy to call for greater investment. This may include public fundraising, raising contributions from individual donors to the effort to develop a new generation of contraceptive options.
A third priority is generating interest in contraceptive research from scientists and innovators. As such, awareness of the need for new options and the market potential for new products needs to permeate the research community. Researchers need to see career paths in the field and so this also involves demonstrating clear pathways for discoveries to move from early science to the market. The Male Contraceptive Initiative and other groups are already undertaking efforts to support and mentor young innovators and scientists interested in contraception; NewGen would build on these efforts to deepen human capital within the field through its own catalytic grant-making.
Our fourth priority is supporting innovations in reaching the marketplace. The founders of NewGen have seen first-hand how working from a patient or user perspective can be an important platform from which to bring users, researchers, clinicians, providers, innovators, regulators, payors and investors together to address barriers to innovation. Users have a strong interest in seeing more improved contraceptive options, but they also bear the risks of safety failures. User support and insights can be helpful for innovators, regulators and payors making decisions on product design, approval and reimbursement. NewGen aims to conduct research on user needs and preferences and support forums for cross-sectoral collaboration on topics such as barriers to contraceptive R&D.
Sidebar: Commentary on novelty
We use “novel” to indicate a major step in a different direction from existing products. When it comes to drugs or new devices, there are several layers to examine for novelty:
- Does the new drug or device have a new mechanism of action? A challenge in contraception is that the vast majority of the higher efficacy options, the hormonal methods, are variants on the same mechanism of action. In addition, because the two main active ingredients, estrogens and progestins, are hormones that work at multiple places along the reproductive signaling system (the hypothalamic-pituitary-gonadal axis), they have pleiotropic biological effects not necessarily core to their contraceptive actions (Rivera et al., 1999).
- Is the new product an entirely new molecular entity (NME) or new device? i.e. is it a completely new class of NMEs or is it a close family- member of an existing class? The first estrogen or progestin in the early oral contraceptives would be considered first-in-class NMEs, but first-in- class compounds are followed by variants with small chemical differences that fall into the same molecular family or class. They have different chemical structures so they are still called NMEs. Estrogens and progestins can each be seen as major classes that have subfamilies. Progestin or estrogen NMEs that emerge with substantial chemical and profile differences create new subclasses. Related to this is the concept of “generations”. For example, the progestin, segesterone acetate, that was approved by the FDA in August 2018, was sufficiently different from prior NMEs that it was designated a fourth- generation progestin. Such innovations on the original NMEs are important because they create variants with different properties such as potency, longevity, and higher specificity which can sometimes lead to a narrower side-effect profile, for instance by reducing the pleiotropic effects of hormones (Kumar et al., 2000). Variants of NMEs typically work on the same mechanism-of-action, so they don’t offer the inherent novelty of new molecules or devices with a different mechanism of action.
- Is the drug or device an extension of existing technologies with the same molecular entity or fundamental device as existing products? Existing NMEs that have proven safety and efficacy are critical tools, and typically it is possible to optimize a product that contains an NME after its initial launch. This optimization can be divided into two groups. First, products with an existing ME(s) but different doses or combinations. Second, products with an existing ME(s) but with a new administration form or route10, or a more significant advance in drug delivery. For instance, delivery advances such as films, contraceptive patches, rings and implants.11
Hormonal contraceptives have seen a high level of variation in category 2, especially in the progestins. There are now many progestin variants that attempt to narrow the wide mechanism of action and pleiotropic effects in progestins (Edwards & Can, 2023).
Hormonal contraceptives have had extensive innovation (probably more than any other group of drugs) in the third category. Within category 3, the more complex the change, and the more innovative relative to existing products, the more R&D investment required. However, almost always these changes need considerably less R&D investment than a new class of NMEs with a new mechanism of action, and typically considerably less than a new molecular entity in an existing class because any changes in the chemistry of a drug have the potential to change the efficacy and side effect profiles so require more extensive testing. A major driver of the lower cost of category 3 innovations is that, because they typically have fewer unknowns, they have a much higher probability of success than programs with a higher level of novelty.
Innovation focused on iteration of existing methods and their active ingredients is critical and can represent important advances for patients: The Annovera® contraceptive ring can now offer a full years’ protection to users and a very high degree of user autonomy. It combines innovation on existing NMEs (a new progestin, segesterone acetate) and on delivery technology; Mirena® was a ground-breaking product that ushered in the hormonal class of IUDs. These IUDs could be considered a combination of two existing methods with innovation in the IUD component and in the site of delivery of progestin. Both Annovera® and Mirena® required intensive scientific investment and deserve to be considered “novel”. However, other category 2 and 3 innovations may bring marginal improvements for patients.
Combinations of category 2 and 3 innovation on hormonals are responsible for most of the 285 different contraceptives marketed in the U.S. today. Hormonal contraceptives come in many dosages, combinations, administration and delivery options – there are pills, rings, sponges, IUDs, injectables and implants containing different mixtures of different progestins and estrogens. This increases the chance that someone will find a hormonal contraceptive that fits their needs.
But we also need entirely novel approaches with new mechanisms of action because there are features we can’t necessarily engineer out of, or in to, the existing technologies. Phexxi®, which was approved for contraceptive use in 2020, is one of the first recent contraceptives with a novel mechanism of action (vaginal pH reduction which inhibits sperm motility). But at a pregnancy rate of 21 people out of a 100 in the first year of use, it has lower effectiveness levels than many of the most popular contraceptives, see Figure 1 (Bradley et al., 2023). By contrast, in 2022, 62% of all new launches in other therapeutic areas were first in class, with many new mechanisms of action (Aitken, Murray et al., 2023).
So when we use “novel” in this paper, we are open to innovation in each layer, but we seek innovations that truly create a major advance for users on the prior products. Given over six decades of optimization of hormonal methods, we feel that we need a strong push on innovating on new mechanisms of action. It’s time for more diverse contraceptive options.
8 The absolute risk of a disease describes a person’s risk of developing that disease over a defined period of time.
9 Relative risk compares the risk of two different groups of people with different exposures (e.g., those using CHC versus those not using CHCs) and describes how much that exposure changes your risk of disease.
10 Forms of administration are variations like pills or gel tablets or films. The route is how it is administered, e.g. IV versus oral.
11 While we have focused on pharmaceuticals extensions here, analogous extensions of devices such as IUDs can be found – on a spectrum between tweaks to an existing device to substantive re-engineering.
Glossary

Glossary of terms and abbreviations
Terms
Access: The ease with which individuals can obtain needed medical services, including contraceptives. It often involves considerations of availability, affordability, and acceptability of healthcare services.
Amenorrhea: The absence of menstruation. It can be a side effect of some contraceptive methods and may be seen as a benefit or a negative depending on individual preferences.
Barrier Methods: Contraceptives that physically prevent sperm from reaching the egg, including condoms, diaphragms, and cervical caps.
Clinical Trials: Research studies performed in people that are aimed at evaluating a medical, surgical, or behavioral intervention. They are the primary way researchers find out if a new treatment, like a new drug or diet or medical device, is safe and effective in people.
Combined Hormonal Contraceptives (CHCs): Methods that contain both estrogen and progestin, such as combined oral contraceptives (COCs), contraceptive patches, and vaginal rings. These methods suppress ovulation and thicken cervical mucus to prevent fertilization.
Efficacy and effectiveness: Efficacy is typically used to describe a product’s ability to prevent pregnancy under clinical trial conditions. Effectiveness is under normal conditions of use. The following effectiveness category definitions come from Contraceptive Technology 22nd edition (Bradley et al., 2019; Cason et al., 2023). Based on first year of use pregnancy rates Category 1: < 1 pregnancy per 100 women in 1 year with either perfect or typical use. Category 2: 1-7 pregnancies per 100 women in 1 year with typical use. Category 3: More than 8 pregnancies per 100 women in 1 year with typical use.
Emergency Contraceptive Pills (ECPs): Pills taken after unprotected sex to prevent pregnancy. Common types include ulipristal acetate (UPA), levonorgestrel (LNG), and the ‘Yuzpe regimen’ which uses a higher dose of regular birth control pills.
Fertility Awareness-Based Methods (FABMs): Contraceptive methods that involve tracking menstrual cycles and identifying fertile days to avoid unprotected sex during those times.
Hormonal Intrauterine Devices (IUDs): T-shaped devices inserted into the uterus that release progestin to prevent pregnancy. They are effective for 3-7 years depending on the product.
Implants: Thin rods placed under the skin that release progestin. They are effective for up to three years.
Injectables: Progestin-only contraceptives administered via injection every three months.
Lactational Amenorrhea Method (LAM): A temporary contraceptive method that relies on the natural infertility that occurs during breastfeeding. It is effective for up to six months postpartum if certain conditions are met.
Long-Acting Reversible Contraceptives (LARCs): Contraceptives that provide effective protection for an extended period without requiring user action, including IUDs and implants.
Non-Hormonal Methods: Contraceptives that do not use hormones to prevent pregnancy, such as copper IUDs, barrier methods, and FABMs.
Novel: See “sidebar on novelty” for how NewGen uses this term.
Oral Contraceptives: Pills taken daily that contain hormones (either combined estrogen and progestin or progestin-only) to prevent ovulation and fertilization.
Ovulation: The release of an egg from the ovary, which can be prevented by certain contraceptive methods.
Pelvic Inflammatory Disease (PID): An infection of the female reproductive organs that can be a rare complication of IUD insertion.
Perfect Use: The effectiveness of a contraceptive method when used consistently and correctly every time. Contrasted to Typical Use: The effectiveness of a contraceptive method under typical conditions, including inconsistent or incorrect use. For example, the male condom has a typical use failure rate of about 13% compared to 2% when used perfectly.
pH: a measure of how acid (or basic) something is – relevant to contraception because making the vagina more acidic reduces sperm motility
Preclinical Studies: Laboratory and animal studies that test new drugs, procedures, or treatments before they are tried in people to evaluate their safety, effectiveness, and potential side effects.
Progestin-Only Contraceptives (POCs): Methods that contain only progestin, including progestin-only pills (POPs), injectables, implants, and hormonal IUDs.
Progestin-Only Pills (POPs): Methods that contain only progestin in pill form. Spermicides: Chemicals that immobilize or kill sperm, often used in conjunction with barrier methods for increased effectiveness.
Target Identification: The process of identifying molecular targets that could be used for new drug development.
Target Validation: The process of confirming that identified targets are directly involved in a disease process and can be targeted effectively by a drug.
Tubal Ligation: A permanent surgical procedure for female sterilization in which the fallopian tubes are closed or blocked.
Typical Use: The effectiveness of a contraceptive method under typical conditions, including inconsistent or incorrect use. For example, the male condom has a typical use failure rate of about 13% compared to 2% when used perfectly. Contrasted to Perfect Use: The effectiveness of a contraceptive method when used consistently and correctly every time.
Ulipristal Acetate (UPA): An emergency contraceptive pill that is available by prescription in the United States and over-the-counter in Europe.
Vasectomy: A permanent surgical procedure for male sterilization in which the vas deferens are closed or blocked to prevent sperm from being included in ejaculate.
Withdrawal (Coitus Interruptus): A contraceptive method in which the penis is withdrawn from the vagina before ejaculation to prevent sperm from entering the reproductive tract.
Abbreviations
ACOG: American College of Obstetricians and Gynecologists CHC: Combined Hormonal Contraceptives
COC: Combined Oral Contraceptives
Cu-IUD: Copper Intrauterine Device
DMPA: Depot Medroxyprogesterone Acetate (Depo-Provera) DVT: Deep Vein Thrombosis
EC: Emergency Contraception
ECP: Emergency Contraceptive Pill
FABM: Fertility Awareness-Based Method FDA: Food and Drug Administration
HIV: Human Immunodeficiency Virus IUD: Intrauterine Device
LAM: Lactational Amenorrhea Method
LNG: Levonorgestrel
LNG-IUD: Levonorgestrel-Releasing Intrauterine Device LARC: Long-Acting Reversible Contraceptive
MEC: Medical Eligibility Criteria
MPT: Multipurpose Prevention Technology
NME New Molecular Entity
NSFG: National Survey of Family Growth
OCP: Oral Contraceptive Pills
PID: Pelvic Inflammatory Disease
POC: Progestin-Only Contraceptive
POP: Progestin-Only Pills
R&D: Research and Development
STI: Sexually Transmitted Infection
UPA: Ulipristal Acetate
VTE: Venous Thromboembolism
Bibliography

3Daughters. (2023). 3Daughters website. https://www.3daughtershealth.com/about-us/
Agénor, M., Cottrill, A. A., Kay, E., Janiak, E., Gordon, A. R., & Potter, J. (2020). Contraceptive Beliefs, Decision Making and Care Experiences Among Transmasculine Young Adults: A Qualitative Analysis. Perspectives on Sexual and Reproductive Health, 52(1), 7–14. https://doi.org/10.1363/psrh.12128
Aitken, M., Connelly, N., Kleinrock, M., & Pritchett, Jamie. (2023, February 15). Global Trends in R&D 2023—IQVIA. Iqvia. https://www.iqvia.com/insights/the-iqvia-institute/reports- and-publications/reports/global-trends-in-r-and-d-2023
Aitken, M., Kleinrock, M., Campanelli, G., Tawil, C., & Vokey, M. (2020). Medicine Spending and Affordability in the U.S. https://www.iqvia.com/insights/the-iqvia- institute/reports/medicine-spending-and-affordability-in-the-us
Aitken, Murray, Kleinrock, Michael, & Simorellis, Alana. (2023, April 23). The Changing Landscape of Research and Development. Iqvia. https://www.iqvia.com/insights/the- iqvia-institute/reports/the-changing-landscape-of-research-and-development
Aitken, Murray, Pritchett, Jamie, & Kleinrock, Michael. (2022, April 21). The Use of Medicines in the U.S. 2022. Iqvia. https://www.iqvia.com/insights/the-iqvia-institute/reports/the- use-of-medicines-in-the-us-2022
Ali, M., & Tran, N. T. (2022). Defining counselling in contraceptive information and services: Outcomes from an expert think tank. BMJ Sexual & Reproductive Health, 48(2), 79–81. https://doi.org/10.1136/bmjsrh-2021-201132
Allen, R. H., Cwiak, C. A., & Kaunitz, A. M. (2013). Contraception in women over 40 years of age. CMAJ, 185(7), 565–573. https://doi.org/10.1503/cmaj.121280
Altuntaş, E., Tekko, I. A., Vora, L. K., Kumar, N., Brodsky, R., Chevallier, O., McAlister, E., Kurnia Anjani, Q., McCarthy, H. O., & Donnelly, R. F. (2022). Nestorone nanosuspension-loaded dissolving microneedles array patch: A promising novel approach for “on-demand” hormonal female-controlled peritcoital contraception. International Journal of Pharmaceutics, 614, 121422. https://doi.org/10.1016/j.ijpharm.2021.121422
American Chemical Society. (2022, March 23). A non-hormonal pill could soon expand men’s birth control options. American Chemical Society. https://www.acs.org/pressroom/newsreleases/2022/march/non-hormonal-pill-could- soon-expand-mens-birth-control-options.html
American College of Obstetricians and Gynecologists (ACOG). (2022). Committee Statement: Patient-Centered Contraceptive Counseling. Obstetrics & Gynecology, 139(2), 4.
Anderson, D. J. (2019). Population and the Environment—Time for Another Contraception Revolution. New England Journal of Medicine, 381(5), 397–399. https://doi.org/10.1056/NEJMp1906733
Anderson, D. J., Politch, J. A., Cone, R. A., Zeitlin, L., Lai, S. K., Santangelo, P. J., Moench, T. R., & Whaley, K. J. (2020). Engineering monoclonal antibody-based contraception and multipurpose prevention technologies†. Biology of Reproduction, 103(2), 275–285. https://doi.org/10.1093/biolre/ioaa096
Artiga, S., Hill, L., Orgera, K., & Damico, A. (2021, July 16). Health Coverage by Race and Ethnicity, 2010-2019. Kaiser Family Foundation (KFF). https://www.kff.org/racial-equity- and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity/
Balbach, M., Fushimi, M., Huggins, D. J., Steegborn, C., Meinke, P. T., Levin, L. R., & Buck, J. (2020). Optimization of lead compounds into on-demand, nonhormonal contraceptives: Leveraging a public-private drug discovery institute collaboration†. Biology of Reproduction, 103(2), 176–182. https://doi.org/10.1093/biolre/ioaa052
Baldeon-Vaca, G., Marathe, J. G., Politch, J. A., Mausser, E., Pudney, J., Doud, J., Nador, E., Zeitlin, L., Pauly, M., Moench, T. R., Brennan, M., Whaley, K. J., & Anderson, D. J. (2021). Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine, 69, 103478. https://doi.org/10.1016/j.ebiom.2021.103478
Baram, I., Aharon, A., Klein, R., & Shkolnik, K. (2020). Real-world experience with the IUB Ballerine MIDI copper IUD: An observational, single-centre study in Israel. The European Journal of Contraception & Reproductive Health Care: The Official Journal of the European Society of Contraception, 25(1), 49–53. https://doi.org/10.1080/13625187.2019.1699048
Barot, S. (2013a). In Search of Breakthroughs: Renewing Support for Contraceptive Research and Development. 16(1), 7.
Barot, S. (2013b, March 22). In Search of Breakthroughs: Renewing Support for Contraceptive Research and Development. Guttmacher Institute. https://doi.org/10.1363/2013.13403
Bayer AG. (2022, October 20). Bayer signs grant agreement to advance innovation in non- hormonal contraception. Bayer. https://www.bayer.com/media/en-us/bayer-signs- grant-agreement-to-advance-innovation-in-non-hormonal-contraception/
Behre, H. M., Zitzmann, M., Anderson, R. A., Handelsman, D. J., Lestari, S. W., McLachlan, R. I., Meriggiola, M. C., Misro, M. M., Noe, G., Wu, F. C. W., Festin, M. P. R., Habib, N. A., Vogelsong, K. M., Callahan, M. M., Linton, K. A., & Colvard, D. S. (2016). Efficacy and Safety of an Injectable Combination Hormonal Contraceptive for Men. The Journal of Clinical Endocrinology & Metabolism, 101(12), 4779–4788. https://doi.org/10.1210/jc.2016-2141
Berglas, N. F., Kimport, K., Mays, A., Kaller, S., & Biggs, M. A. (2021). “It’s Worked Well for Me”: Young Women’s Reasons for Choosing Lower-Efficacy Contraceptive Methods. Journal of Pediatric and Adolescent Gynecology, 34(3), 341–347. https://doi.org/10.1016/j.jpag.2020.12.012
Bianchi, E., Doe, B., Goulding, D., & Wright, G. J. (2014). Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature, 508(7497), Article 7497. https://doi.org/10.1038/nature13203
BIO Industry Organization. (2022). How long does it take to get a drug approved? | BIO. https://www.bio.org/blogs/how-long-does-it-take-get-drug-approved
Bitzer, J., Rapkin, A., & Soares, C. N. (2018). Managing the risks of mood symptoms with LNG- IUS: A clinical perspective. The European Journal of Contraception & Reproductive Health Care, 23(5), 321–325. https://doi.org/10.1080/13625187.2018.1521512
Blithe, D. L. (2016). Pipeline for contraceptive development. Fertility and Sterility, 106(6), 1295– 1302. https://doi.org/10.1016/j.fertnstert.2016.07.1115
Bonnington, A., Dianat, S., Kerns, J., Hastings, J., Hawkins, M., De Haan, G., & Obedin-Maliver, J. (2020). Society of Family Planning clinical recommendations: Contraceptive counseling for transgender and gender diverse people who were female sex assigned at birth.
Contraception, 102(2), 70–82. https://doi.org/10.1016/j.contraception.2020.04.001
Borrero, S., Nikolajski, C., Steinberg, J. R., Freedman, L., Akers, A. Y., Ibrahim, S., & Schwarz, E. B. (2015). “It just happens”: A qualitative study exploring low-income women’s perspectives on pregnancy intention and planning. Contraception, 91(2), 150–156. https://doi.org/10.1016/j.contraception.2014.09.014
Boyce, T. M., & Neiterman, E. (2021). Women in larger bodies’ experiences with contraception: A scoping review. Reproductive Health, 18(1), 89. https://doi.org/10.1186/s12978-021- 01139-2
Brache, V., Vieira, C. S., Plagianos, M., Lansiaux, M., Merkatz, R., Sussman, H., Cochon, L., Tejada, A. S., Kumar, N., Loeven, D., Blithe, D. L., Aprem, A. S., Williams, A. R., Kannan, A., Bagchi, I. C., & Sitruk-Ware, R. (2021). Pharmacodynamics and pharmacokinetics of a copper intrauterine contraceptive system releasing ulipristal acetate: A randomized proof-of-concept study. Contraception, 104(4), 327–336. https://doi.org/10.1016/j.contraception.2021.06.010
Bradley, S. E. K., Polis, C. B., Bankole, A., & Croft, T. (2019). Global Contraceptive Failure Rates: Who Is Most at Risk? Studies in Family Planning, 50(1), 3–24. https://doi.org/10.1111/sifp.12085
Bradley, S. E. K., Polis, C. B., Micks, E., & Steiner, M. J. (2023). Effectiveness, safety, and comparative side effects. In Contraceptive Technology (22nd ed.). Jones & Bartlett Learning.
Brown, A. (2022, June 7). About 5% of young adults in the U.S. say their gender is different from their sex assigned at birth. Pew Research Center. https://www.pewresearch.org/short- reads/2022/06/07/about-5-of-young-adults-in-the-u-s-say-their-gender-is-different- from-their-sex-assigned-at-birth/
Brown, Amy, & Elmhurst, Edwin. (2023, April 21). Big pharma holds steady on research spending. Evaluate.Com. https://www.evaluate.com/vantage/articles/insights/other- data/big-pharma-holds-steady-research-spending
Burger, Ludwig, & Weiss, Patricia. (2023, March 24). Bayer says drug research focus no longer on women’s health | Reuters. Https://Www.Reuters.Com/. https://www.reuters.com/business/healthcare-pharmaceuticals/bayer-says-drug-research-focus-no-longer-womens-health-2023-03-24/
Burke, A. E. (2011). The state of hormonal contraception today: Benefits and risks of hormonal
contraceptives: progestin-only contraceptives. American Journal of Obstetrics and
Gynecology, 205(4), S14–S17. https://doi.org/10.1016/j.ajog.2011.04.033
Burke, K. L., Potter, J. E., & White, K. (2020). Unsatisfied contraceptive preferences due to cost
among women in the United States. Contraception: X, 2, 100032.
https://doi.org/10.1016/j.conx.2020.100032
Cahill, E. P., & Blumenthal, P. D. (2018). Pericoital contraception. Current Opinion in Obstetrics
& Gynecology, Publish Ahead of Print. https://doi.org/10.1097/GCO.0000000000000491
Cahill, E. P., Lerma, K., Shaw, K. A., & Blumenthal, P. D. (2022). Potential candidate for oral pericoital contraception: Evaluating ulipristal acetate plus cyclo-oxygenase-2 inhibitor for ovulation disruption. BMJ Sexual & Reproductive Health, 48(3), 217–221. https://doi.org/10.1136/bmjsrh-2021-201446
Cairns-Smith, S., Jaffe, H. K., & Speidel, J. J. (2024). Contraceptive technology is failing to meet the needs of people in the U.S. because of under-investment in new methods. Contraception, 0(0). https://doi.org/10.1016/j.contraception.2024.110518
Callahan, R. L., Mehta, N. J., Nanda, K., & Kopf, G. S. (2020). The new contraceptive revolution: Developing innovative products outside of industry†,‡. Biology of Reproduction, 103(2), 157–166. https://doi.org/10.1093/biolre/ioaa067
Carlson, E. J., Francis, R., Liu, Y., Li, P., Lyon, M., Santi, C. M., Hook, D. J., Hawkinson, J. E., & Georg, G. I. (2022). Discovery and Characterization of Multiple Classes of Human CatSper Blockers. Chemmedchem, 17(15), e202000499. https://doi.org/10.1002/cmdc.202000499
Cason, P., Cwiak, C., Edelman, A., Kowal, D., Marrazzo, J., & Nelson, A. (2023). Contraceptive Technology (22nd ed.). Jones & Bartlett Learning.
CDC. (2021, January 25). STI Prevalence, Incidence, and Cost Estimates Infographic. Centers for Disease Control and Prevention. https://www.cdc.gov/std/statistics/prevalence-2020- at-a-glance.htm
CDC. (2022, March 16). Incidence, Prevalence, and Cost of Sexually Transmitted Infections in the United States. https://www.cdc.gov/nchhstp/newsroom/fact-sheets/std/STI-Incidence- Prevalence-Cost-Factsheet.html
Centers for Disease Control and Prevention. (2019, October 6). CDC - Combined Hormonal Contraceptives—US SPR - Reproductive Health. https://www.cdc.gov/reproductivehealth/contraception/mmwr/spr/combined.html
Centers for Disease Control and Prevention. (2022). Sexually Transmitted Disease Surveillance: Preliminary 2021 Data. US Department of Health and Human Services. https://www.cdc.gov/std/statistics/2021/default.htm
Chamberlain, S. G., Vogelsong, K. M., Weinberger, M., Serazin, E., Cairns-Smith, S., & Gerrard, S. E. (2020). Reboot contraceptives research—It has been stuck for decades. Nature, 587(7835), 543–545. https://doi.org/10.1038/d41586-020-03287-0
Chang, Z., Qin, W., Zheng, H., Schegg, K., Han, L., Liu, X., Wang, Y., Wang, Z., McSwiggin, H., Peng, H., Yuan, S., Wu, J., Wang, Y., Zhu, S., Jiang, Y., Nie, H., Tang, Y., Zhou, Y., Hitchcock, M. J. M., ... Yan, W. (2021). Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nature Communications, 12(1), Article 1. https://doi.org/10.1038/s41467-021-21517-5
Charlton, B. M., Everett, B. G., Light, A., Jones, R. K., Janiak, E., Gaskins, A. J., Chavarro, J. E., Moseson, H., Sarda, V., & Austin, S. B. (2020). Sexual Orientation Differences in Pregnancy and Abortion Across the Lifecourse. Women’s Health Issues: Official Publication of the Jacobs Institute of Women’s Health, 30(2), 65–72. https://doi.org/10.1016/j.whi.2019.10.007
Chesson, H. W., Dunne, E. F., Hariri, S., & Markowitz, L. E. (2014). The Estimated Lifetime Probability of Acquiring Human Papillomavirus in the United States. Sexually Transmitted Diseases, 41(11), 660–664. https://doi.org/10.1097/OLQ.0000000000000193
Christofield, M. (2018, October 24). Could precision medicine help women choose the contraceptive that works best for them? STAT. https://www.statnews.com/2018/10/24/precision-medicine-contraceptive-choice/
Chung, S. S. W., Wang, X., & Wolgemuth, D. J. (2016). Prolonged Oral Administration of a Pan- Retinoic Acid Receptor Antagonist Inhibits Spermatogenesis in Mice With a Rapid Recovery and Changes in the Expression of Influx and Efflux Transporters. Endocrinology, 157(4), 1601–1612. https://doi.org/10.1210/en.2015-1675
Cirqle Biomedical. (2022). Home Page. Cirqle Biomedical. https://www.cirqle.bio
Cohen, J., Shull, D., & Reed, S. (2023). Co-delivery of an HIV prophylactic and contraceptive
using PGSU as a long-acting multipurpose prevention technology. Expert Opinion on
Drug Delivery, 20(2), 285–299. https://doi.org/10.1080/17425247.2023.2168642
Coleman-Minahan, K., Ela, E. J., White, K., & Grossman, D. (2021). Contraindications to Hormonal Contraception Among Postpartum Women in Texas. Obstetrics and Gynecology, 137(5), 907–915. https://doi.org/10.1097/AOG.0000000000004347
Commissioner, O. of the. (2023, July 13). FDA Approves First Nonprescription Daily Oral
Contraceptive. FDA; FDA. https://www.fda.gov/news-events/press-announcements/fda-
approves-first-nonprescription-daily-oral-contraceptive
Committee on New Frontiers in Contraceptive Research, Board on Health Sciences Policy, & Institute of Medicine. (2004). New Frontiers in Contraceptive Research: A Blueprint for Action (S. J. Nass & J. F. Strauss, Eds.; p. 10905). National Academies Press. https://doi.org/10.17226/10905
Contraceptive Deserts 2024 | Power to Decide. (2024). https://powertodecide.org/what-we- do/contraceptive-deserts
Contraline, Inc. (2022). Open-label, Single Arm, Multi-Centre, Prospective First-in-Human Study to Assess the Safety of the ADAMTM System (Clinical Trial Registration NCT05134428). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT05134428
Coombe, J., Harris, M. L., & Loxton, D. (2016). What qualities of long-acting reversible contraception do women perceive as desirable or undesirable? A systematic review. Sexual Health, 13(5), 404. https://doi.org/10.1071/SH15189
Curtis, K. M., Tepper, N. K., Jatlaoui, T. C., Berry-Bibee, E., Horton, L. G., Zapata, L. B., Simmons, K. B., Pagano, H. P., Jamieson, D. J., & Whiteman, M. K. (2016). U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. Morbidity and Mortality Weekly Report: Recommendations and Reports, 65(3), 1–103.
Cwiak, C. (2020). Contraception for high risk patients. Seminars in Perinatology, 44(5), 151268. https://doi.org/10.1016/j.semperi.2020.151268
Cwiak, C., Gellasch, T., & Zieman, M. (2004). Peripartum contraceptive attitudes and practices. Contraception, 70(5), 383–386. https://doi.org/10.1016/j.contraception.2004.05.010
Dalessandro, C., Thorpe, R., & Sanders, J. (2022). “I Just Don’t Think I Can Deal:” Contraceptive Method Acceptability, Dealbreakers, and Women’s Embodied Sense of Self. Sexuality Research and Social Policy. https://doi.org/10.1007/s13178-022-00744-5
Dam, A., Yeh, P. T., Burke, A. E., & Kennedy, C. E. (2022). Contraceptive values and preferences of pregnant women, postpartum women, women seeking emergency contraceptives, and women seeking abortion services: A systematic review. Contraception, 111, 39–47. https://doi.org/10.1016/j.contraception.2021.10.007
Dama Health. (n.d.). Dama Health—Find your fit. Dama Health. Retrieved December 13, 2022, from https://damahealth.com/
Daniels, K., & Abma, J. (2020). NCHS Data Brief: Current Contraceptive Status Among Women Aged 15–49: United States, 2017–2019 (p. 8). U.S. Department of Health and Human Services.
Danna, K., Angel, A., Kuznicki, J., Lemoine, L., Lerma, K., & Kalamar, A. (2021). Leveraging the Client-Provider Interaction to Address Contraceptive Discontinuation: A Scoping Review of the Evidence That Links Them. Global Health: Science and Practice, 9(4), 948–963. https://doi.org/10.9745/GHSP-D-21-00235
Danvers, A. A., & Evans, T. A. (2022). Risk of Sterilization Regret and Age: An Analysis of the National Survey of Family Growth, 2015-2019. Obstetrics and Gynecology, 139(3), 433– 439. https://doi.org/10.1097/AOG.0000000000004692
Daré Bioscience. (2023). DARE-LARC1 [Daré Bioscience]. https://darebioscience.com/pipeline/dare-larc1/
Dehlendorf, C., Grumbach, K., Schmittdiel, J. A., & Steinauer, J. (2017). Shared Decision Making in Contraceptive Counseling. Contraception, 95(5), 452–455. https://doi.org/10.1016/j.contraception.2016.12.010
Dehlendorf, C., Park, S. Y., Emeremni, C. A., Comer, D., Vincett, K., & Borrero, S. (2014). Racial/ethnic disparities in contraceptive use: Variation by age and women’s reproductive experiences. American Journal of Obstetrics and Gynecology, 210(6), 526.e1-526.e9. https://doi.org/10.1016/j.ajog.2014.01.037
Dehlendorf, C., Reed, R., Fox, E., Seidman, D., Hall, C., & Steinauer, J. (2018). Ensuring our research reflects our values: The role of family planning research in advancing reproductive autonomy. Contraception, 98(1), 4–7. https://doi.org/10.1016/j.contraception.2018.03.015
Delvaux, T., Jespers, V., Benova, L., & van de Wijgert, J. (2021). Acceptability and Satisfaction of Contraceptive Vaginal Rings in Clinical Studies: A Systematic Review and Narrative Synthesis. 2, 14.
Derby, N., Lal, M., Aravantinou, M., Kizima, L., Barnable, P., Rodriguez, A., Lai, M., Wesenberg, A., Ugaonkar, S., Levendosky, K., Mizenina, O., Kleinbeck, K., Lifson, J. D., Peet, M. M., Lloyd, Z., Benson, M., Heneine, W., O’Keefe, B. R., Robbiani, M., ... Zydowsky, T. M. (2018). Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nature Communications, 9(1), Article 1. https://doi.org/10.1038/s41467-018-06349-0
Dhont, M. (2010). History of oral contraception. The European Journal of Contraception & Reproductive Health Care, 15(sup2), S12–S18. https://doi.org/10.3109/13625187.2010.513071
Diedrich, J. T., Desai, S., Zhao, Q., Secura, G., Madden, T., & Peipert, J. F. (2015). Association of short-term bleeding and cramping patterns with long-acting reversible contraceptive method satisfaction. American Journal of Obstetrics and Gynecology, 212(1), 50.e1- 50.e8. https://doi.org/10.1016/j.ajog.2014.07.025
Donnelly, K. Z., Foster, T. C., & Thompson, R. (2014). What matters most? The content and concordance of patients’ and providers’ information priorities for contraceptive decision making. Contraception, 90(3), 280–287. https://doi.org/10.1016/j.contraception.2014.04.012
Dorman, E., Perry, B., Polis, C. B., Campo-Engelstein, L., Shattuck, D., Hamlin, A., Aiken, A., Trussell, J., & Sokal, D. (2018). Modeling the impact of novel male contraceptive methods on reductions in unintended pregnancies in Nigeria, South Africa, and the United States. Contraception, 97(1), 62–69. https://doi.org/10.1016/j.contraception.2017.08.015
Drugs.com. (2023, April 12). List of Contraceptives + Types & Side Effects. Drugs.Com. https://www.drugs.com/drug-class/contraceptives.html
Edwards, M., & Can, A. S. (2023). Progestin. In StatPearls. StatPearls Publishing.
http://www.ncbi.nlm.nih.gov/books/NBK563211/
Eli Health. (2023). Eli Health. Eli Health. https://eli.health/
Elias, C., & Coggins, C. (2001). Acceptability Research on Female-Controlled Barrier Methods to
Prevent Heterosexual Transmission of HIV: Where Have We Been? Where Are We Going? Journal of Women’s Health & Gender-Based Medicine, 10(2), 163–173. https://doi.org/10.1089/152460901300039502
Envision Intelligence. (2022, November 7). Global Contraceptives Market—Size, Outlook, Trends and Forecasts (2019 – 2025). Envision Intelligence. https://www.envisioninteligence.com/industry-report/global-contraceptives-market/
Ersek, J. L., Brunner Huber, L. R., Thompson, M. E., & Warren-Findlow, J. (2011). Satisfaction and Discontinuation of Contraception by Contraceptive Method Among University Women. Maternal and Child Health Journal, 15(4), 497–506. https://doi.org/10.1007/s10995-010-0610-y
EU Clinical Trials Register. (2023, November 11). Clinical Trials register—Search for mifepristone. https://www.clinicaltrialsregister.eu/ctr- search/search?query=mifepristone
Evofem Biosciences. (2022). Pipeline In Development. Evofem Biosciences. https://www.evofem.com/pipeline/in-development/
Femasys Inc. (2023, September 27). Femasys Inc. Expands FemBloc Pivotal Trial Enrollment Securing First Academic Site to Participate Femasys Inc. https://ir.femasys.com/news/news-details/2023/Femasys-Inc.-Expands-FemBloc- Pivotal-Trial-Enrollment-Securing-First-Academic-Site-to-Participate/default.aspx
Fey, Rachel, & McDonald-Mosley, Raegan. (2022, April). When Your Birth Control Isn’t Covered | Power to Decide. Https://Powertodecide.Org/. https://powertodecide.org/what-we- do/information/resource-library/when-your-birth-control-isnt-covered
FHI 360. (2023a). Calliope, the Contraceptive Pipeline Database. CTI Exchange. https://pipeline.ctiexchange.org/
FHI 360. (2023b). Development of a Biodegradable Implant for Contraception. FHI 360. https://www.fhi360.org/projects/development-biodegradable-implant-contraception
Finer, L. B., & Henshaw, S. K. (2006). Disparities in Rates of Unintended Pregnancy in the United States, 1994 and 2001. Perspectives on Sexual and Reproductive Health, 38(2), 90–96.
Finer, L. B., & Zolna, M. R. (2016). Declines in Unintended Pregnancy in the United States, 2008– 2011. New England Journal of Medicine, 374(9), 843–852. https://doi.org/10.1056/NEJMsa1506575
Fowler, C. I., Ahrens, K. A., Decker, E., Gable, J., Wang, J., Frederiksen, B., Loyola Briceño, A. C., & Moskosky, S. B. (2019). Patterns and trends in contraceptive use among women attending Title X clinics and a national sample of low-income women. Contraception: X, 1, 100004. https://doi.org/10.1016/j.conx.2019.100004
Frank, Richard. G., & Hannick, K. (2022, June 2). Five things to understand about pharmaceutical R&D. Brookings. https://www.brookings.edu/articles/five-things-to-understand-about- pharmaceutical-rd/
Frederiksen, B., & Ahrens, K. (2020). Understanding the extent of contraceptive non-use among women at risk of unintended pregnancy, National Survey of Family Growth 2011–2017. Contraception: X, 2, 100033. https://doi.org/10.1016/j.conx.2020.100033
Frederiksen, B., Ranji, U., Apr 21, M. L. P., & 2021. (2021, April 21). Women’s Sexual and Reproductive Health Services: Key Findings from the 2020 KFF Women’s Health Survey. KFF. https://www.kff.org/womens-health-policy/issue-brief/womens-sexual-and- reproductive-health-services-key-findings-from-the-2020-kff-womens-health-survey/
Friedland, B. A., Mathur, S., & Haddad, L. B. (2021). The Promise of the Dual Prevention Pill: A Framework for Development and Introduction. Frontiers in Reproductive Health, 3. https://www.frontiersin.org/articles/10.3389/frph.2021.682689
Frost, J. J., Singh, S., & Finer, L. B. (2007). Factors Associated with Contraceptive Use and Nonuse, United States, 2004. Perspectives on Sexual and Reproductive Health, 39(2), 90– 99. https://doi.org/10.1363/3909007
Frost, J. J., Sonfield, A., Zolna, M. R., & Finer, L. B. (2014). Return on Investment: A Fuller Assessment of the Benefits and Cost Savings of the US Publicly Funded Family Planning Program. The Milbank Quarterly, 92(4), 696–749. https://doi.org/10.1111/1468- 0009.12080
Frost, J. J., Zolna, M. R., Frohwirth, L. F., Douglas-Hall, A., Blades, N., Mueller, J., Pleasure, Z. H., & Kochhar, S. (2019). Publicly Supported Family Planning Services in the United States: Likely Need, Availability and Impact, 2016 (p. 52). Guttmacher Institute.
Fruzzetti, F., Fidecicchi, T., Montt Guevara, M. M., & Simoncini, T. (2021). Estetrol: A New Choice for Contraception. Journal of Clinical Medicine, 10(23), 5625. https://doi.org/10.3390/jcm10235625
Fuentes, L., Ingerick, M., Jones, R., & Lindberg, L. (2018). Adolescents’ and Young Adults’ Reports of Barriers to Confidential Health Care and Receipt of Contraceptive Services. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 62(1), 36–43. https://doi.org/10.1016/j.jadohealth.2017.10.011
Geist, C., Aiken, A. R., Sanders, J. N., Everett, B. G., Myers, K., Cason, P., Simmons, R. G., & Turok, D. K. (2020). Beyond intent: Exploring the association of contraceptive choice with questions about Pregnancy Attitudes, Timing and How important is pregnancy prevention (PATH) questions. 12.
Gemmill, A. (2018). Perceived Subfecundity and Contraceptive Use Among Young Adult U.S. Women: Perceived subfecundity and contraceptive use. Perspectives on Sexual and Reproductive Health, 50(3), TK.
Getting behind human-centered design for contraceptive innovation. (2018, December 26). R&E Search for Evidence. https://researchforevidence.fhi360.org/getting-behind-human- centered-design-for-contraceptive-innovation
Gilliam, M. L., Neustadt, A., Whitaker, A., & Kozloski, M. (2011). Familial, Cultural and Psychosocial Influences of Use of Effective Methods of Contraception among Mexican- American Adolescents and Young Adults. Journal of Pediatric and Adolescent Gynecology, 24(2), 79–84. https://doi.org/10.1016/j.jpag.2010.10.002
Girum, T., & Wasie, A. (2018). Return of fertility after discontinuation of contraception: A systematic review and meta-analysis. Contraception and Reproductive Medicine, 3(1), 9. https://doi.org/10.1186/s40834-018-0064-y
Glasier, A. (2002). Implantable contraceptives for women: Effectiveness, discontinuation rates, return of fertility, and outcome of pregnancies. Contraception, 65(1), 29–37. https://doi.org/10.1016/S0010-7824(01)00284-0
Glasier, A. (2010). Acceptability of contraception for men: A review. Contraception, 82(5), 453– 456. https://doi.org/10.1016/j.contraception.2010.03.016
Glasier, A., Bhattacharya, S., Evers, H., Gemzell-Danielsson, K., Hardman, S., Heikinheimo, O., La Vecchia, C., Somigliana, E., & Annual Capri Workshop Group. (2019). Contraception after pregnancy. Acta Obstetricia Et Gynecologica Scandinavica, 98(11), 1378–1385. https://doi.org/10.1111/aogs.13627
Glasier, A. F., Anakwe, R., Everington, D., Martin, C. W., van der Spuy, Z., Cheng, L., Ho, P. C., & Anderson, R. A. (2000). Would women trust their partners to use a male pill? Human Reproduction (Oxford, England), 15(3), 646–649. https://doi.org/10.1093/humrep/15.3.646
Godfrey, E. M., Zapata, L. B., Cox, C. M., Curtis, K. M., & Marchbanks, P. A. (2016). Unintended pregnancy risk and contraceptive use among women 45-50 years old: Massachusetts, 2006, 2008, and 2010. American Journal of Obstetrics and Gynecology, 214(6), 712.e1- 712.e8. https://doi.org/10.1016/j.ajog.2015.12.006
Gomez, A. M., Arteaga, S., Aronson, N., Goodkind, M., Houston, L., & West, E. (2020). No Perfect Method: Exploring How Past Contraceptive Methods Influence Current Attitudes Toward Intrauterine Devices. Archives of Sexual Behavior, 49(4), 1367–1378. https://doi.org/10.1007/s10508-019-1424-7
Grady, C. D., Dehlendorf, C., Cohen, E. D., Schwarz, E. B., & Borrero, S. (2015). Racial and ethnic differences in contraceptive use among women who desire no future children, 2006– 2010 National Survey of Family Growth. Contraception, 92(1), 62–70. https://doi.org/10.1016/j.contraception.2015.03.017
Greene Foster, D. (2020). The Turnaway Study: Ten years, a thousand women, and the consequences of having—Or being denied—An abortion. Scribner.
Grunloh, D. S., Casner, T., Secura, G. M., Peipert, J. F., & Madden, T. (2013). Characteristics Associated With Discontinuation of Long-Acting Reversible Contraception Within the First 6 Months of Use. Obstetrics and Gynecology (New York. 1953), 122(6), 1214–1221. https://doi.org/10.1097/01.AOG.0000435452.86108.59
Gurr, B. (2011). Complex Intersections: Reproductive Justice and Native American Women: Complex Intersections. Sociology Compass, 5(8), 721–735. https://doi.org/10.1111/j.1751-9020.2011.00400.x
Guttmacher Institute. (2016, March 14). Emergency Contraception. Guttmacher Institute. https://www.guttmacher.org/state-policy/explore/emergency-contraception
Guttmacher Institute. (2020a, January 22). Contraceptive Use in the United States by
Demographics. Guttmacher Institute. https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states
Guttmacher Institute. (2020b, June 10). Unintended Pregnancy and Abortion Worldwide. Guttmacher Institute. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
Guttmacher Institute. (2021, April 7). Contraceptive Use in the United States by Method. Guttmacher Institute. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states
Guttmacher Institute. (2023, July 1). Emergency Contraception. Guttmacher Institute.
https://www.guttmacher.org/state-policy/explore/emergency-contraception
Haddad, L. B., RamaRao, S., Hazra, A., Birungi, H., & Sailer, J. (2021). Addressing contraceptive needs exacerbated by COVID-19: A call for increasing choice and access to self-managed methods. Contraception, 103(6), 377–379. https://doi.org/10.1016/j.contraception.2021.03.023
Haddad, L. B., Townsend, J. W., & Sitruk-Ware, R. (2021). Contraceptive Technologies: Looking Ahead to New Approaches to Increase Options for Family Planning. Clinical Obstetrics and Gynecology, 64(3), 435–448. https://doi.org/10.1097/GRF.0000000000000628
Hales, C. M., Fryar, C. D., Carroll, M. D., Freedman, D. S., & Ogden, C. L. (2018). Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA, 319(16), 1723–1725. https://doi.org/10.1001/jama.2018.3060
Halpern, V., Brache, V., Taylor, D., Lendvay, A., Cochón, L., Jensen, J. T., & Dorflinger, L. J. (2021). Clinical trial to evaluate pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after subcutaneous administration of Depo-Provera. Fertility and Sterility, 115(4), 1035–1043. https://doi.org/10.1016/j.fertnstert.2020.11.002
Hanna, C. B., Mudaliar, D., John, K., Allen, C. L., Sun, L., Hawkinson, J. E., Schönbrunn, E., Georg, G. I., & Jensen, J. T. (2020). Development of WEE2 kinase inhibitors as novel non- hormonal female contraceptives that target meiosis†. Biology of Reproduction, 103(2), 368–377. https://doi.org/10.1093/biolre/ioaa097
Hatcher, R., Nelson, A., Trussell, J., Cwiak, C., Cason, P., Policar, M., Edelman, A., Aiken, A., Marrazzo, J., & Kowal, D. (2018). Contraceptive Technology, 21st Edition. Ayer Company Publishers, Inc.
Health Decisions. (2021). A Multi-center, Single-blind, Randomized Clinical Trial to Compare Two Copper IUDs: Mona Lisa NT Cu380 Mini and ParaGard (Clinical Trial Registration NCT03124160). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03124160
Heise, L. L. (1997). Beyond Acceptability: Reorienting Research on Contraceptive Choice. 9.
HeraHealthSolutions. (n.d.). Home. HeraHealthSolutions. Retrieved February 2, 2023, from https://www.herahealthsolutions.com
Herman, J. L., Flores, A. R., & O’Neill, K. K. (2022). How many adults and youth identify as transgender in the United States? (p. 26). Williams Institute at UCLA School of Law. https://williamsinstitute.law.ucla.edu/wp-content/uploads/Trans-Pop-Update-Jun- 2022.pdf
HHS, DOL, and Treasury Issue Guidance Regarding Birth Control Coverage | CMS. (2022, July 28). CMS.Gov. https://www.cms.gov/newsroom/press-releases/hhs-dol-and-treasury- issue-guidance-regarding-birth-control-coverage
Higgins, J. A., Ryder, K., Skarda, G., Koepsel, E., & Bennett, E. A. (2015). The Sexual Acceptability of Intrauterine Contraception: A Qualitative Study of Young Adult Women: IUDs’ sexual acceptability. Perspectives on Sexual and Reproductive Health, 47(3), 115–122.
https://doi.org/10.1363/47e4515
Higgins, J. A., & Smith, N. K. (2016). The Sexual Acceptability of Contraception: Reviewing the Literature and Building a New Concept. The Journal of Sex Research, 53(4–5), 417–456. https://doi.org/10.1080/00224499.2015.1134425
Holt, K., Reed, R., Crear-Perry, J., Scott, C., Wulf, S., & Dehlendorf, C. (2020). Beyond same-day long-acting reversible contraceptive access: A person-centered framework for advancing high-quality, equitable contraceptive care. American Journal of Obstetrics and Gynecology, 222(4), S878.e1-S878.e6. https://doi.org/10.1016/j.ajog.2019.11.1279
Home—ClinicalTrials.gov. (As accessed). https://clinicaltrials.gov/ct2/home
Hoopes, A. J., Teal, S. B., Akers, A. Y., & Sheeder, J. (2018). Low Acceptability of Certain Contraceptive Methods among Young Women. Journal of Pediatric and Adolescent Gynecology, 31(3), 274–280. https://doi.org/10.1016/j.jpag.2017.11.008
Hooton, A. (2005). Symposium: A Broader Vision of the Reproductive Rights Movement: Fusing Mainstream and Latina Feminism. Social Policy, 13, 29.
Hoppes, E., Nwachukwu, C., Hennegan, J., Blithe, D. L., Cordova-Gomez, A., Critchley, H., Doncel, G. F., Dorflinger, L. J., Haddad, L. B., Mackenzie, A. C. L., Maybin, J. A., Moley, K., Nanda, K., Sales Vieira, C., Vwalika, B., Kibira, S. P. S., Mickler, A., OlaOlorun, F. M., Polis, C. B., ... Rountree, L. (2022). Global research and learning agenda for building evidence on contraceptive-induced menstrual changes for research, product development, policies, and programs. Gates Open Research, 6, 49. https://doi.org/10.12688/gatesopenres.13609.1
Hubacher, D., Schreiber, C. A., Turok, D. K., Jensen, J. T., Creinin, M. D., Nanda, K., White, K. O., Dayananda, I., Teal, S. B., Chen, P.-L., Chen, B. A., Goldberg, A. B., Kerns, J. L., Dart, C., Nelson, A. L., Thomas, M. A., Archer, D. F., Brown, J. E., Castaño, P. M., ... Blithe, D. L. (2022). Continuation rates of two different-sized copper intrauterine devices among nulliparous women: Interim 12-month results of a single-blind, randomised, multicentre trial. EClinicalMedicine, 51, 101554. https://doi.org/10.1016/j.eclinm.2022.101554
Hubacher, D., Spector, H., Monteith, C., & Chen, P.-L. (2018). Not seeking yet trying long-acting reversible contraception: A 24-month randomized trial on continuation, unintended pregnancy and satisfaction. Contraception, 97(6), 524–532.
https://doi.org/10.1016/j.contraception.2018.02.001
Initiative for Multipurpose Prevention Technology (IMPT). (2022, June). MPT Product Development Database. Initiative for Multipurpose Prevention Technology (IMPT). https://mpts101.org/wp/wp-content/uploads/2022/06/MPT-Pipeline-Table_The-IMPT- June-2022_Indication-Combination.pdf
Inne. (2023). Inne | Cycle- & Ovulation Tracker | Progesterone Saliva-Test. https://www.inne.io/en/home
Innovation Equity Forum. (2023). Women’s Health Innovation Opportunity Map 2023: 50 High- Return Opportunities to Advance Global Women’s Health R&D. Bill & Melinda Gates Foundation and US National Institutes of Health. https://orwh.od.nih.gov/sites/orwh/files/docs/womens-health-rnd-opportunity- map_2023_508.pdf
Inoue, K., Barratt, A., & Richters, J. (2015). Does research into contraceptive method discontinuation address women’s own reasons? A critical review. 8.
Insurance Coverage of Contraceptives. (2021). https://www.guttmacher.org/print/state- policy/explore/insurance-coverage-contraceptives
Insurance Coverage of Contraceptives. (2022, May 1). Guttmacher Institute. https://www.guttmacher.org/state-policy/explore/insurance-coverage-contraceptives
Interest Among U.S. Men for New Male Contraceptive Options: Consumer Research Study. (2019). Male Contraceptive Initiative. https://www.malecontraceptive.org/uploads/1/3/1/9/131958006/mci_consumerresear chstudy_5.pdf
International Partnership for Microbicides, Inc. (2022). A Randomized, Double-Blind, Phase 1b Study in Healthy HIV-Negative Women to Evaluate the Pharmacokinetics, Safety, and Bleeding Patterns Associated With 90-Day Use of Core-Sheath Vaginal Rings Releasing Dapivirine and Levonorgestrel (Clinical Trial Registration NCT05041699). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT0504169
IQVIA. (2022). Global Trends in R&D 2022. The IQVIA Institute. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-trends-in-r-and-d- 2022
Iuliano, D. A., Speizer, I. S., Santelli, J., & Kendall, C. (2006). Reasons for Contraceptive Nonuse at First Sex and Unintended Pregnancy. American Journal of Health Behavior, 30(1), 92– 102. https://doi.org/10.5993/AJHB.30.1.9
Jackson, A. V., Karasek, D., Dehlendorf, C., & Foster, D. G. (2016). Racial and ethnic differences in women’s preferences for features of contraceptive methods. Contraception, 93(5), 406–411. https://doi.org/10.1016/j.contraception.2015.12.010
Jacobs, J., & Stanfors, M. (2013). Racial and ethnic differences in U.S. women’s choice of reversible contraceptives, 1995-2010. Perspectives on Sexual and Reproductive Health, 45(3), 139–147. https://doi.org/10.1363/4513913
Jakkaraj, S. R., Young, V. G., & Georg, G. I. (2018). Syntheses of PDE3A inhibitor ORG9935 and determination of the absolute stereochemistries of its enantiomers by X-ray crystallography. Tetrahedron, 74(22), 2769–2774. https://doi.org/10.1016/j.tet.2018.04.045
Jensen, J. T. (2015). Present and future contraception: Does discovery of targets lead to new contraceptives? Expert Opinion on Therapeutic Targets, 19(11), 1429–1432. https://doi.org/10.1517/14728222.2015.1039939
Johnson-Mallard, V., Kostas-Polston, E. A., Woods, N. F., Simmonds, K. E., Alexander, I. M., & Taylor, D. (2017). Unintended pregnancy: A framework for prevention and options for midlife women in the US. Women’s Midlife Health, 3, 8. https://doi.org/10.1186/s40695-017-0027-5
Johnston, D. S., & Kopf, G. S. (2023). The urgent need for innovation in contraception. Biology of Reproduction, 108(4), 519–521. https://doi.org/10.1093/biolre/ioad020
Jones, J. M. (2021, February 24). LGBT Identification Rises to 5.6% in Latest U.S. Estimate. Gallup.Com. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest- estimate.aspx
Jones, J., Mosher, W., & Daniels, K. (2012). Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. National Health Statistics Reports, 60, 1–25.
Jones, N., Marks, R., Ramirez, R., & Rios-Vargas, M. (2021, August 12). Improved Race and Ethnicity Measures Reveal U.S. Population Is Much More Multiracial. US Census Bureau. https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures- reveal-united-states-population-much-more-multiracial.html
Jones, R. K. (2018). Is pregnancy fatalism normal? An attitudinal assessment among women trying to get pregnant and those not using contraception. Contraception, 98(4), 255– 259. https://doi.org/10.1016/j.contraception.2018.05.015
Jones, R. K., Purcell, A., Singh, S., & Finer, L. B. (2005). Adolescents’ Reports of Parental Knowledge of Adolescents’ Use of Sexual Health Services and Their Reactions to Mandated Parental Notification for Prescription Contraception. JAMA, 293(3), 340–348. https://doi.org/10.1001/jama.293.3.340
Kakaiya, R., Lopez, L. L., & Nelson, A. L. (2017). Women’s perceptions of contraceptive efficacy and safety. Contraception and Reproductive Medicine, 2, 19. https://doi.org/10.1186/s40834-017-0046-5
Kavanaugh, M. L., & Pliskin, E. (2020). Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F&S Reports, 1(2), 83–93. https://doi.org/10.1016/j.xfre.2020.06.006
Kavanaugh, M. L., Pliskin, E., & Hussain, R. (2022). Associations between unfulfilled contraceptive preferences due to cost and low-income patients’ access to and experiences of contraceptive care in the United States, 2015–2019. Contraception: X, 4, 100076. https://doi.org/10.1016/j.conx.2022.100076
KFF Women’s Health team. (2022, August 4). Emergency Contraception. KFF. https://www.kff.org/womens-health-policy/fact-sheet/emergency-contraception/
Kiattiburut, W., Zhi, R., Lee, S. G., Foo, A. C., Hickling, D. R., Keillor, J. W., Goto, N. K., Li, W., Conlan, W., Angel, J. B., Wang, G., & Tanphaichitr, N. (2018). Antimicrobial peptide LL-37 and its truncated forms, GI-20 and GF-17, exert spermicidal effects and microbicidal
activity against Neisseria gonorrhoeae. Human Reproduction (Oxford, England), 33(12), 2175–2183. https://doi.org/10.1093/humrep/dey315
Killick, S. R., Leary, C., Trussell, J., & Guthrie, K. A. (2011). Sperm content of pre-ejaculatory fluid. Human Fertility, 14(1), 48–52. https://doi.org/10.3109/14647273.2010.520798
Kirtane, A. R., Hua, T., Hayward, A., Bajpayee, A., Wahane, A., Lopes, A., Bensel, T., Ma, L., Stanczyk, F. Z., Brooks, S., Gwynne, D., Wainer, J., Collins, J., Tamang, S. M., Langer, R., & Traverso, G. (2019). A once-a-month oral contraceptive. Science Translational Medicine, 11(521), eaay2602. https://doi.org/10.1126/scitranslmed.aay2602
Kortsmit, K. (2021). Abortion Surveillance—United States, 2019. MMWR. Surveillance Summaries, 70. https://doi.org/10.15585/mmwr.ss7009a1
Kramer, R. D., Higgins, J. A., Everett, B., Turok, D. K., & Sanders, J. N. (2022). A prospective analysis of the relationship between sexual acceptability and contraceptive satisfaction over time. American Journal of Obstetrics and Gynecology, 226(3), 396.e1-396.e11. https://doi.org/10.1016/j.ajog.2021.10.008
Kreitzer, R. J., Smith, C. W., Kane, K. A., & Saunders, T. M. (2021). Affordable but Inaccessible? Contraception Deserts in the US States. Journal of Health Politics, Policy and Law, 46(2), 277–304. https://doi.org/10.1215/03616878-8802186
Krempasky, C., Harris, M., Abern, L., & Grimstad, F. (2020). Contraception across the transmasculine spectrum. American Journal of Obstetrics & Gynecology, 222(2), 134– 143. https://doi.org/10.1016/j.ajog.2019.07.043
Kumar, N., Koide, S. S., Tsong, Y., & Sundaram, K. (2000). Nestorone: A progestin with a unique pharmacological profile. Steroids, 65(10–11), 629–636. https://doi.org/10.1016/s0039- 128x(00)00119-7
Lauring, J. R., Lehman, E. B., Deimling, T. A., Legro, R. S., & Chuang, C. H. (2016). Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. American Journal of Obstetrics and Gynecology, 215(3), 330.e1-330.e7. https://doi.org/10.1016/j.ajog.2016.03.047
Lawton, A., & George, B. (2018, December 26). Getting behind human-centered design for contraceptive innovation. FHI 360: R&E Search for Evidence. https://researchforevidence.fhi360.org/getting-behind-human-centered-design-for-contraceptive-innovation
Le Guen, M., Schantz, C., Régnier-Loilier, A., & de La Rochebrochard, E. (2021). Reasons for rejecting hormonal contraception in Western countries: A systematic review. Social Science & Medicine, 284, 114247. https://doi.org/10.1016/j.socscimed.2021.114247
Lehrer, J. A., Pantell, R., Tebb, K., & Shafer, M.-A. (2007). Forgone Health Care among U.S. Adolescents: Associations between Risk Characteristics and Confidentiality Concern. Journal of Adolescent Health, 40(3), 218–226. https://doi.org/10.1016/j.jadohealth.2006.09.015
Lessard, L. N., Karasek, D., Ma, S., Darney, P., Deardorff, J., Lahiff, M., Grossman, D., & Foster, D. G. (2012). Contraceptive Features Preferred by Women At High Risk of Unintended Pregnancy. Perspectives on Sexual and Reproductive Health, 44(3), 194–200. https://doi.org/10.1363/4419412
Li, L., Gatto, G. J., Brand, R. M., Krovi, S. A., Cottrell, M. L., Norton, C., van der Straten, A., & Johnson, L. M. (2021). Long-acting biodegradable implant for sustained delivery of antiretrovirals (ARVs) and hormones. Journal of Controlled Release: Official Journal of the Controlled Release Society, 340, 188–199. https://doi.org/10.1016/j.jconrel.2021.10.021
Li, W., Tang, J., Terry, R. N., Li, S., Brunie, A., Callahan, R. L., Noel, R. K., Rodríguez, C. A., Schwendeman, S. P., & Prausnitz, M. R. (2019). Long-acting reversible contraception by effervescent microneedle patch. Science Advances, 5(11), eaaw8145. https://doi.org/10.1126/sciadv.aaw8145
Light, A., Wang, L.-F., Zeymo, A., & Gomez-Lobo, V. (2018). Family planning and contraception use in transgender men. Contraception, 98(4), 266–269. https://doi.org/10.1016/j.contraception.2018.06.006
Littlejohn, K. E. (2012). Hormonal Contraceptive Use and Discontinuation Because of Dissatisfaction: Differences by Race and Education. 20.
Long, J. E., Lee, M. S., & Blithe, D. L. (2021). Update on Novel Hormonal and Nonhormonal Male Contraceptive Development. The Journal of Clinical Endocrinology & Metabolism, 106(6), e2381–e2392. https://doi.org/10.1210/clinem/dgab034
Madden, T., Secura, G. M., Nease, R. F., Politi, M. C., & Peipert, J. F. (2015). The role of contraceptive attributes in women’s contraceptive decision making. American Journal of Obstetrics and Gynecology, 213(1), 46.e1-46.e6. https://doi.org/10.1016/j.ajog.2015.01.051
Mahony, H., Spinner, C., Vamos, C. A., & Daley, E. M. (2021). Social Network Influences on Young Women’s Choice to Use Long-Acting Reversible Contraception: A Systematic Review. Journal of Midwifery & Women’s Health, 66(6), 758–771. https://doi.org/10.1111/jmwh.13280
Male Contraceptive Initiative. (n.d.). Non-Hormonal Reversible Male Contraception (NHMRC) Pipeline Database. Male Contraceptive Initiative. Retrieved December 11, 2022, from https://www.malecontraceptive.org/nhrmc-database.html
Mansour, D., Gemzell-Danielsson, K., Inki, P., & Jensen, J. T. (2011). Fertility after discontinuation of contraception: A comprehensive review of the literature. Contraception, 84(5), 465–477. https://doi.org/10.1016/j.contraception.2011.04.002
Marnach, M. L., Gave, C. J., & Casey, P. M. (2020). Contraceptive Challenges in Women With Common Medical Conditions. Mayo Clinic Proceedings, 95(11), 2525–2534. https://doi.org/10.1016/j.mayocp.2020.08.045
Marshall, C., Guendelman, S., Mauldon, J., & Nuru-Jeter, A. (2016). Young Women’s Contraceptive Decision Making: Do Preferences for Contraceptive Attributes Align with Method Choice?: Contraceptive attributes preferences and method use. Perspectives on Sexual and Reproductive Health, 48(3), 119–127. https://doi.org/10.1363/48e10116
MedinCell. (2022). Additional US$ 4 million received for next development steps of MedinCell’s 6-month active injectable bioresorbable subcutaneous contraceptive. MedinCell. https://www.medincell.com/en/2022/11/30/additional-us-4-million-received-for-next- development-steps-of-medincells-6-month-active-injectable-bioresorbable- subcutaneous-contraceptive/
Meier, S., Sundstrom, B., Delay, C., & DeMaria, A. L. (2021). “Nobody’s Ever Told Me That:” Women’s Experiences with Shared Decision-making when Accessing Contraception. Health Communication, 36(2), 179–187. https://doi.org/10.1080/10410236.2019.1669271
Mensch, B. S., van der Straten, A., & Katzen, L. L. (2012). Acceptability in microbicide and PrEP trials: Current status and a reconceptualization. Current Opinion in HIV and AIDS, 7(6), 534–541. https://doi.org/10.1097/COH.0b013e3283590632
Mikulic, Matej. (2023, October 24). Worldwide pharmaceutical R&D spending 2014-2028. Statista. https://www.statista.com/statistics/309466/global-r-and-d-expenditure-for- pharmaceuticals/
Miller, T. A., Allen, R. H., Kaunitz, A. M., & Cwiak, C. A. (2018). Contraception for midlife women: A review. Menopause (New York, N.Y.), 25(7), 817–827. https://doi.org/10.1097/GME.0000000000001073
Minnis, A. M., Shiboski, S. C., & Padian, N. S. (2003). Barrier Contraceptive Method Acceptability and Choice Are Not Reliable Indicators of Use: Sexually Transmitted Diseases, 30(7), 556–561. https://doi.org/10.1097/00007435-200307000-00005
Moreau, C., Cleland, K., & Trussell, J. (2007a). Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception, 76(4), 267–272.
Moreau, C., Cleland, K., & Trussell, J. (2007b). Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception, 76(4), 267–272.
https://doi.org/10.1016/j.contraception.2007.06.008
Moreira, L. R., Ewerling, F., Barros, A. J. D., & Silveira, M. F. (2019). Reasons for nonuse of contraceptive methods by women with demand for contraception not satisfied: An assessment of low and middle-income countries using demographic and health surveys. Reproductive Health, 16(1), 148. https://doi.org/10.1186/s12978-019-0805-7
Moseson, H., Fix, L., Hastings, J., Stoeffler, A., Lunn, M. R., Flentje, A., Lubensky, M. E., Capriotti, M. R., Ragosta, S., Forsberg, H., & Obedin-Maliver, J. (2021). Pregnancy intentions and outcomes among transgender, nonbinary, and gender-expansive people assigned female or intersex at birth in the United States: Results from a national, quantitative survey. International Journal of Transgender Health, 22(1–2), 30–41. https://doi.org/10.1080/26895269.2020.1841058
Mosher, W., Jones, J., & Abma, J. (2015). Nonuse of contraception among women at risk of unintended pregnancy in the United States. Contraception, 92(2), 170–176. https://doi.org/10.1016/j.contraception.2015.05.004
National Center for Health Statistics. (2019). Table 26. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States, selected years 1988-1994 through 2015-2018. https://www.cdc.gov/nchs/data/hus/2019/026- 508.pdf
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (U.S.). Division of STD Prevention. (2019). Sexually transmitted disease surveillance 2018. CDC. https://doi.org/10.15620/cdc.79370
Nelson, A. L., Cohen, S., Galitsky, A., Hathaway, M., Kappus, D., Kerolous, M., Patel, K., & Dominguez, L. (2018). Women’s perceptions and treatment patterns related to contraception: Results of a survey of US women. Contraception, 97(3), 256–273. https://doi.org/10.1016/j.contraception.2017.09.010
Nelson, A. L., Shabaik, S., Xandre, P., Kakaiya, R., Awaida, J., Mellon, M., Schiller, A., & Stohl, H. E. (2019). Perceptions of health risks associated with pregnancy compared to oral contraceptive use. Contraception, 100(3), 193–195. https://doi.org/10.1016/j.contraception.2019.04.008
Nguyen, B. T., Elia, J. L., Ha, C. Y., & Kaneshiro, B. E. (2018). Pregnancy Intention and Contraceptive Use among Women by Class of Obesity: Results from the 2006–2010 and 2011–2013 National Survey of Family Growth. Women’s Health Issues, 28(1), 51–58. https://doi.org/10.1016/j.whi.2017.09.010
NICHD. (2022). Spotlight: One year and counting: Male birth control study reaches milestone. NICHD. https://www.nichd.nih.gov/newsroom/news/080222-NEST
Oddens, B. J. (1999). Women’s satisfaction with birth control: A population survey of physical and psychological effects of oral contraceptives, intrauterine devices, condoms, natural family planning, and sterilization among 1466 women. Contraception, 59(5), 277–286. https://doi.org/10.1016/S0010-7824(99)00034-7
O’Rand, M. G., Hamil, K. G., Adevai, T., & Zelinski, M. (2018). Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PloS One, 13(4), e0195953. https://doi.org/10.1371/journal.pone.0195953
Organon. (2022, July 28). Organon and Cirqle Biomedical Enter Research Collaboration and License Agreement for Investigational Non-hormonal, On-demand Contraceptive Candidate. Organon. https://www.organon.com/news/organon-and-cirqle-biomedical- enter-research-collaboration-and-license-agreement-for-investigational-non-hormonal- on-demand-contraceptive-candidate/
Pantarhei. (2023). Pantarhei Bioscience pipeline: ARC. Pantarhei Bioscience. https://www.pantarheibio.com/pipeline/
Parsemus Foundation. (2023). Vasalgel Male Contraceptive. Parsemus Foundation. https://www.parsemus.org/humanhealth/male-contraceptive-research/vasalgel-male- contraceptive/
Paying for Contraception in the United States. (2020, January 23). Guttmacher Institute. https://www.guttmacher.org/fact-sheet/paying-contraception-united-states
Pazol, K., Whiteman, M. K., Folger, S. G., Kourtis, A. P., Marchbanks, P. A., & Jamieson, D. J. (2015). Sporadic contraceptive use and nonuse: Age-specific prevalence and associated factors. American Journal of Obstetrics and Gynecology, 212(3), 324.e1-324.e8. https://doi.org/10.1016/j.ajog.2014.10.004
Peluffo, M. C., Stanley, J., Braeuer, N., Rotgeri, A., Fritzemeier, K.-H., Fuhrmann, U., Buchmann, B., Adevai, T., Murphy, M. J., Zelinski, M. B., Lindenthal, B., Hennebold, J. D., & Stouffer, R. L. (2014). A prostaglandin E2 receptor antagonist prevents pregnancies during a preclinical contraceptive trial with female macaques. Human Reproduction (Oxford, England), 29(7), 1400–1412. https://doi.org/10.1093/humrep/deu083
Peragallo Urrutia, R., & Polis, C. B. (2019). Fertility awareness based methods for pregnancy prevention. BMJ, l4245. https://doi.org/10.1136/bmj.l4245
Peragallo Urrutia, R., Polis, C. B., Jensen, E. T., Greene, M. E., Kennedy, E., & Stanford, J. B. (2018). Effectiveness of Fertility Awareness–Based Methods for Pregnancy Prevention: A Systematic Review. Obstetrics and Gynecology (New York. 1953), 132(3), 591–604. https://doi.org/10.1097/AOG.0000000000002784
Phexxi®. (2023). Hormone Free Birth Control | Phexxi®. https://www.phexxi.com Plan ATM | Reversible Male Birth Control. (2023, November 12).
Plan A. https://www.planaformen.com
Policy Cures Research. (2024). G-FINDER Data Tool. Policy Cures Research. https://gfinderdata.policycuresresearch.org/pages/data- visualisations/allSexualReproductiveHealthDiseases
Polis, C. B. (2022). Calculations based on data in Table 2 of Frost et al. Perspect Sex Reprod Health 2007;39:90–9.
Polis, C. B., Hussain, R., & Berry, A. (2018). There might be blood: A scoping review on women’s responses to contraceptive-induced menstrual bleeding changes. Reproductive Health, 15(1), 114. https://doi.org/10.1186/s12978-018-0561-0
Polis, C. B., & Jones, R. K. (2018). Multiple contraceptive method use and prevalence of fertility awareness based method use in the United States, 2013–2015. Contraception, 98(3), 188–192. https://doi.org/10.1016/j.contraception.2018.04.013
Polis, C. B., & Zabin, L. S. (2012). Missed Conceptions or Misconceptions: Perceived Infertility Among Unmarried Young Adults In the United States. Perspectives on Sexual and Reproductive Health, 44(1), 30–38. https://doi.org/10.1363/4403012
Population Council. (2015, June 9). Population Council’s Progesterone Contraceptive Vaginal Ring Added to WHO 2015 Essential Medicines List and Included in Medical Eligibility Criteria Guidance for Providers. Population Council. https://www.popcouncil.org/news/population-councils-progesterone-contraceptive- vaginal-ring-added-to-who-20
Population Council. (2021, December 1). Medicines360 and the Population Council Pursue Agreement to Develop Product to Prevent HIV and Unintended Pregnancy for Worldwide Distribution. Population Council. https://www.popcouncil.org/news/medicines360-and- the-population-council-pursue-agreement-to-develop-product
Population Council. (2023). Nonhormonal Contraceptive Multipurpose Prevention Technology Containing Q-Griffithsin – Population Council. https://popcouncil.org/project/non- hormonal-contraceptive-multipurpose-prevention-technology-mpt-containing-q- griffithsin/
PrEPWatch. (2022, October 1). Research Pipeline. PrEPWatch. https://www.prepwatch.org/research-pipeline/
Ramanadhan, S., Jusko, W. J., & Edelman, A. (2020). Pharmacokinetics of Hormonal Contraception in Individuals with Obesity: A Review. Current Obstetrics and Gynecology Reports, 9(2), 72–78. https://doi.org/10.1007/s13669-020-00284-y
Ravindran, T. K. S., Berer, M., Cottingham, J., & World Health Organization. (1997). Beyond acceptability: Users’ perspectives on contraception.
Rennhack, A., Schiffer, C., Brenker, C., Fridman, D., Nitao, E. T., Cheng, Y.-M., Tamburrino, L., Balbach, M., Stölting, G., Berger, T. K., Kierzek, M., Alvarez, L., Wachten, D., Zeng, X.-H., Baldi, E., Publicover, S. J., Kaupp, U. B., & Strünker, T. (2018). A novel cross-species inhibitor to study the function of CatSper Ca2+ channels in sperm. British Journal of Pharmacology, 175(15), 3144–3161. https://doi.org/10.1111/bph.14355
Reproductive Blueprint. (2019, July 1). Blueprint for Sexual and Reproductive Health, Rights, and Justice. Reproductive Blueprint. https://reproblueprint.org/
Research and Development in the Pharmaceutical Industry | Congressional Budget Office. (2021, April 8). Research and Development in the Pharmaceutical Industry. https://www.cbo.gov/publication/57126
Reynolds, C. A., Beccia, A., & Charlton, B. M. (2021). Multiple marginalisation and unintended pregnancy among racial/ethnic and sexual minority college women. Paediatric and Perinatal Epidemiology, 35(4), 493–500. https://doi.org/10.1111/ppe.12744
Rivera, R., Yacobson, I., & Grimes, D. (1999). The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. American Journal of Obstetrics and Gynecology, 181(5 Pt 1), 1263–1269. https://doi.org/10.1016/s0002- 9378(99)70120-1
Roberts, D. (2014). Killing the Black Body: Race, Reproduction, and the Meaning of Liberty. Vintage.
Rominski, S. D., SK Morhe, E., Maya, E., Manu, A., & Dalton, V. K. (2017). Comparing Women’s Contraceptive Preferences With Their Choices in 5 Urban Family Planning Clinics in Ghana. Global Health: Science and Practice, 5(1), 65–74. https://doi.org/10.9745/GHSP- D-16-00281
Rominski, S. D., & Stephenson, R. (2019). Toward a New Definition of Unmet Need for Contraception: Toward a New Definition of Unmet Need for Contraception. Studies in Family Planning, 50(2), 195–198. https://doi.org/10.1111/sifp.12084
Rosenberg, M. J., Waugh, M. S., & Burnhill, M. S. (1998). Compliance, Counseling and Satisfaction with Oral Contraceptives: A Prospective Evaluation. Family Planning Perspectives, 30(2), 89. https://doi.org/10.2307/2991665
Rosenfeld, J. A. (1993). Women’s Satisfaction with Birth Control. The Journal of Family Practice, 36(2), 5.
Ross, J., & Stover, J. (2013). Use of modern contraception increases when more methods become available: Analysis of evidence from 1982-2009. Global Health, Science and Practice, 1(2), 203–212. https://doi.org/10.9745/GHSP-D-13-00010
Rossetti, T., Jackvony, S., Buck, J., & Levin, L. R. (2021). Bicarbonate, carbon dioxide and pH sensing via mammalian bicarbonate-regulated soluble adenylyl cyclase. Interface Focus, 11(2), 20200034. https://doi.org/10.1098/rsfs.2020.0034
Rubens, H. (2022, May 13). Novel Intravaginal Ring as a Non-Hormonal Contraceptive Multipurpose Prevention Technology (MPT). MPT Product Development Database. https://mpts101.org/novel-intravaginal-ring-as-a-non-hormonal-contraceptive-mpt/
Samari, G., Foster, D. G., Ralph, L. J., & Rocca, C. H. (2020). Pregnancy preferences and contraceptive use among US women. Contraception, 101(2), 79–85. https://doi.org/10.1016/j.contraception.2019.10.007
Schaffir, J., Worly, B. L., & Gur, T. L. (2016). Combined hormonal contraception and its effects on mood: A critical review. The European Journal of Contraception & Reproductive Health Care, 21(5), 347–355. https://doi.org/10.1080/13625187.2016.1217327
Schimpf, U., Caldas-Silveira, E., Katchan, L., Vigier-Carriere, C., Lantier, I., Nachmann, G., Gidlöf, S., Jonasson, A. F., Björndahl, L., Trombotto, S., Druart, X., & Crouzier, T. (2022). Topical reinforcement of the cervical mucus barrier to sperm. Science Translational Medicine, 14(673), eabm2417. https://doi.org/10.1126/scitranslmed.abm2417
Schlander, M., Hernandez-Villafuerte, K., Cheng, C.-Y., Mestre-Ferrandiz, J., & Baumann, M. (2021). How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. PharmacoEconomics, 39(11), 1243–1269.
https://doi.org/10.1007/s40273-021-01065-y
Schnyer, A. N., Jensen, J. T., Edelman, A., & Han, L. (2019). Do menstrual cups increase risk of IUD expulsion? A survey of self-reported IUD and menstrual hygiene product use in the United States. The European Journal of Contraception & Reproductive Health Care, 24(5), 368–372. https://doi.org/10.1080/13625187.2019.1643836
Sebela Women’s Health Inc. (2022). A Phase 3, Prospective, Multi-Center, Single-Arm, Open- Label Study to Evaluate VeraCeptTM, a Long-Acting Reversible Intrauterine Contraceptive for Contraceptive Efficacy, Safety, and Tolerability (Clinical Trial Registration NCT03633799). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT03633799
Severy, L. J., & Newcomer, S. (2005). Critical Issues in Contraceptive and STI Acceptability Research. Journal of Social Issues, 61(1), 45–65. https://doi.org/10.1111/j.0022- 4537.2005.00393.x
Shortridge, E., & Miller, K. (2007a). Contraindications to oral contraceptive use among women in the United States, 1999–2001. Contraception, 75(5), 355–360. https://doi.org/10.1016/j.contraception.2006.12.022
Shortridge, E., & Miller, K. (2007b). Contraindications to oral contraceptive use among women in the United States, 1999-2001. Contraception, 75(5), 355–360. https://doi.org/10.1016/j.contraception.2006.12.022
Shrestha, B., Vincent, K., Schaefer, A., Zhu, Y., Vargas, G., Motamedi, M., Swope, K., Morton, J., Simpson, C., Pham, H., Brennan, M. B., Pauly, M. H., Zeitlin, L., Bratcher, B., Whaley, K. J., Moench, T. R., & Lai, S. K. (2021). Hexavalent sperm-binding IgG antibody released from vaginal film for development of potent on-demand nonhormonal female contraception. Proceedings of the National Academy of Sciences, 118(48), e2107832118. https://doi.org/10.1073/pnas.2107832118
Simmons, R. G., Sanders, J. N., Geist, C., Gawron, L., Myers, K., & Turok, D. K. (2019). Predictors of contraceptive switching and discontinuation within the first 6 months of use among Highly Effective Reversible Contraceptive Initiative Salt Lake study participants. Am J Obstet Gynecol, 220, 376.e1-12.
Sitruk-Ware, R. (2006). New progestagens for contraceptive use. Human Reproduction Update, 12(2), 169–178. https://doi.org/10.1093/humupd/dmi046
Sloat, Sarah. (2022, August 12). Birth Control TikTok Is a Symptom of Medicine’s Bigger Problem | WIRED. Wired.Com. https://www.wired.com/story/hormonal-birth-control-tiktok/
Smith, K. (2023, May 4). Women’s health research lacks funding – these charts show how. https://www.nature.com/immersive/d41586-023-01475-2/index.html
Snodgrass, E. (2022, July 30). Mifepristone in Moldova: Women on Waves founder wants to use the abortion pill as contraception in the wake of Roe v. Wade reversal [07-30-2022]. Business Insider. https://www.businessinsider.com/women-on-waves-founder- abortion-pill-roe-v-wade-overturned-2022-6
Soin, K. S., Yeh, P. T., Gaffield, M. E., Ge, C., & Kennedy, C. E. (2022). Health workers’ values and preferences regarding contraceptive methods globally: A systematic review. Contraception, 111, 61–70. https://doi.org/10.1016/j.contraception.2022.04.012
Speidel, J. J., Townsend, J., Williams, E., Quam, J., & Thompson, K. M. J. (2020). Commentary on research to improve contraceptive and multipurpose prevention technologies. Contraception, 101(3), 148–152. https://doi.org/10.1016/j.contraception.2019.12.005
Srikanthan, A., & Reid, R. L. (2008). Religious and Cultural Influences on Contraception. Journal of Obstetrics and Gynaecology Canada, 30(2), 129–137. https://doi.org/10.1016/S1701- 2163(16)32736-0
Steinberg, J. R., Marthey, D., Xie, L., & Boudreaux, M. (2021). Contraceptive method type and satisfaction, confidence in use, and switching intentions. Contraception, 104(2), 176– 182. https://doi.org/10.1016/j.contraception.2021.02.010
Stepanenko, N., Wolk, O., Bianchi, E., Wright, G. J., Schachter-Safrai, N., Makedonski, K., Ouro, A., Ben-Meir, A., Buganim, Y., & Goldblum, A. (2022). In silico Docking Analysis for Blocking JUNO-IZUMO1 Interaction Identifies Two Small Molecules that Block in vitro Fertilization. Frontiers in Cell and Developmental Biology, 10. https://www.frontiersin.org/articles/10.3389/fcell.2022.824629
Stoffel, C., Carpenter, E., Everett, B., Higgins, J., & Haider, S. (2017). Family Planning for Sexual Minority Women. Seminars in Reproductive Medicine, 35(5), 460–468. https://doi.org/10.1055/s-0037-1604456
Stransky, O. M., Wolgemuth, T., Kazmerski, T., Chodoff, A., Borrero, S., & Birru Talabi, M. (2021). Contraception decision-making and care among reproductive-aged women with autoimmune diseases. Contraception, 103(2), 86–91. https://doi.org/10.1016/j.contraception.2020.11.001
Sutton, M. Y., Anachebe, N. F., Lee, R., & Skanes, H. (2021). Racial and Ethnic Disparities in Reproductive Health Services and Outcomes, 2020. Obstetrics and Gynecology, 137(2), 225–233. https://doi.org/10.1097/AOG.0000000000004224
Swan, L. E. T. (2021). The impact of US policy on contraceptive access: A policy analysis. Reproductive Health, 18(1), 235. https://doi.org/10.1186/s12978-021-01289-3
Syeda, S. S., Sánchez, G., Hong, K. H., Hawkinson, J. E., Georg, G. I., & Blanco, G. (2018). Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na,K-ATPase α4 Isoform Inhibitors for Male Contraception. Journal of Medicinal Chemistry, 61(5), 1800–1820. https://doi.org/10.1021/acs.jmedchem.7b00925
Szent-Ivanyi, T. (2023, October 11). Ampel decides to fund research into male contraceptives for the first time. https://www.rnd.de/politik/ampel-beschliesst-erstmals- forschungsfoerderung-fuer-maenner-verhuetungsmittel- UFMYTHN5NFBOLP7TZKSXG7HU5I.html
Taparia, M. (2022, November 2). Pregna’s PPIUD 380A IUD receives WHO/UNFPA PQ. EIN Presswire. https://www.einpresswire.com/article/597115641/pregna-s-ppiud-380a-iud- receives-who-unfpa-pq
Teal, S., & Edelman, A. (2021). Contraception Selection, Effectiveness, and Adverse Effects: A Review. JAMA, 326(24), 2507. https://doi.org/10.1001/jama.2021.21392
Tepper, N. K., Dragoman, M. V., Gaffield, M. E., & Curtis, K. M. (2017). Nonoral combined hormonal contraceptives and thromboembolism: A systematic review. Contraception, 95(2), 130–139. https://doi.org/10.1016/j.contraception.2016.10.005
Teva Branded Pharmaceutical Products R&D, Inc. (2022). A Randomized, Dose-range Finding Study to Evaluate Pharmacokinetics of Medroxyprogesterone Acetate Following a Single Subcutaneous Administration of TV-46046 in Healthy Women of Reproductive Age
(Clinical Trial Registration NCT04682353). clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04682353
The Bill & Melinda Gates Foundation. (2021). Family Planning Strategy Refresh [Presentation to partners]. The Bill & Melinda Gates Foundation.
The White House. (2023a, June 23). FACT SHEET: President Biden Issues Executive Order on Strengthening Access to Contraception. The White House. https://www.whitehouse.gov/briefing-room/statements-releases/2023/06/23/fact- sheet-president-biden-issues-executive-order-on-strengthening-access-to- contraception/
The White House. (2023b, November 13). FACT SHEET: President Joe Biden to Announce First- Ever White House Initiative on Women’s Health Research, An Effort Led by First Lady Jill Biden and the White House Gender Policy Council. The White House. https://www.whitehouse.gov/briefing-room/statements-releases/2023/11/13/fact- sheet-president-joe-biden-to-announce-first-ever-white-house-initiative-on-womens- health-research-an-effort-led-by-first-lady-jill-biden-and-the-white-house-gender-policy- council/
Thirumalai, A., & Amory, J. K. (2021). Emerging approaches to male contraception. Fertility and Sterility, 115(6), 1369–1376. https://doi.org/10.1016/j.fertnstert.2021.03.047
Thirumalai, A., Ceponis, J., Amory, J. K., Swerdloff, R., Surampudi, V., Liu, P. Y., Bremner, W. J., Harvey, E., Blithe, D. L., Lee, M. S., Hull, L., Wang, C., & Page, S. T. (2019). Effects of 28 Days of Oral Dimethandrolone Undecanoate in Healthy Men: A Prototype Male Pill. The Journal of Clinical Endocrinology and Metabolism, 104(2), 423–432. https://doi.org/10.1210/jc.2018-01452
Thompson, K., & Speidel, J. J. (2014). Public and philanthropic funding started the contraceptive revolution and will be needed to finish it (p. 5). Bixby Center for Global Reproductive Health, University of California, San Francisco. https://bixbycenter.ucsf.edu/sites/bixbycenter.ucsf.edu/files/Funding_contraceptive_R %26D.pdf
Thurman, A. R., Brache, V., Cochon, L., Ouattara, L. A., Chandra, N., Jacot, T., Yousefieh, N., Clark, M. R., Peet, M., Hanif, H., Schwartz, J. L., Ju, S., Marzinke, M. A., Erikson, D. W., Parikh, U., Herold, B. C., Fichorova, R. N., Tolley, E., & Doncel, G. F. (2022). Randomized, placebo controlled phase I trial of the safety, pharmacokinetics, pharmacodynamics and acceptability of a 90 day tenofovir plus levonorgestrel vaginal ring used continuously or cyclically in women: The CONRAD 138 study. PLOS ONE, 17(10), e0275794. https://doi.org/10.1371/journal.pone.0275794
Ti, A., Soin, K., Rahman, T., Dam, A., & Yeh, P. T. (2022). Contraceptive values and preferences of adolescents and young adults: A systematic review. Contraception, 111, 22–31. https://doi.org/10.1016/j.contraception.2021.05.018
Tietze, C., & Bongaarts, J. (1975). Fertility rates and abortion rates: Simulations of family limitation. Studies in Family Planning, 6(5), 114–120.
Tolley, E. E., Zissette, S., Taylor, J., Hanif, H., Ju, S., Schwarz, J., Thurman, A., Tyner, D., Brache, V., & Doncel, G. F. (2022). Acceptability of a Long-Acting, Multipurpose Vaginal Ring: Findings from a Phase I Trial in the U.S. and Dominican Republic. Journal of Women’s Health, jwh.2021.0394. https://doi.org/10.1089/jwh.2021.0394
Tong, Y. W., Lo, S. S. T., Fung, B. W. K., Cameron, S. T., Ng, E. H. Y., & Li, R. H. W. (2022). Acceptability of different mechanisms of action of contraception in women: A questionnaire survey. BMJ Sexual & Reproductive Health, 48(2), 117–122. https://doi.org/10.1136/bmjsrh-2021-201110
Trau, H. A., Brännström, M., Curry, T. E., & Duffy, D. M. (2016). Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Human Reproduction (Oxford, England), 31(2), 436–444. https://doi.org/10.1093/humrep/dev320
Turok, D., Gero, A., Simmons, R., Kaiser, J., Stoddard, G., Sexsmith, C., Gawron, L., & Sanders, J. (2022). Levonorgestrel vs. Copper Intrauterine Devices for Emergency Contraception. New England Journal of Medicine, 384. https://www.nejm.org/doi/full/10.1056/NEJMoa2022141
Turok, D. K., Nelson, A. L., Dart, C., Schreiber, C. A., Peters, K., Schreifels, M. J., Katz, B., & VeraCept Phase 2 Clinical Investigator Group. (2020). Efficacy, Safety, and Tolerability of a New Low-Dose Copper and Nitinol Intrauterine Device: Phase 2 Data to 36 Months. Obstetrics and Gynecology, 135(4), 840–847. https://doi.org/10.1097/AOG.0000000000003756
Ueda, P., Mercer, C. H., Ghaznavi, C., & Herbenick, D. (2020). Trends in Frequency of Sexual Activity and Number of Sexual Partners Among Adults Aged 18 to 44 Years in the US, 2000-2018. JAMA Network Open, 3(6), e203833. https://doi.org/10.1001/jamanetworkopen.2020.3833
Vahdat, H. L., Shane, K., & Nickels, L. M. (2020). The role of team science in the future of male contraception†. Biology of Reproduction, 103(2), 167–175. https://doi.org/10.1093/biolre/ioaa086
Van Heertum, K., & Liu, J. (2017). Contraception and conception in Mid-life: A review of the current literature. Women’s Midlife Health, 3(1), 3. https://doi.org/10.1186/s40695- 017-0022-x
van Lunsen, R. H. W., Zimmerman, Y., Coelingh Bennink, H. J. T., Termeer, H. M. M., Appels, N., Fauser, B. C. J. M., & Laan, E. (2018). Maintaining physiologic testosterone levels during combined oral contraceptives by adding dehydroepiandrosterone: II. Effects on sexual function. A phase II randomized, double-blind, placebo-controlled study. Contraception, 98(1), 56–62. https://doi.org/10.1016/j.contraception.2018.02.014
van Ryn, M., Burgess, D. J., Dovidio, J. F., Phelan, S. M., Saha, S., Malat, J., Griffin, J. M., Fu, S. S., & Perry, S. (2011). THE IMPACT OF RACISM ON CLINICIAN COGNITION, BEHAVIOR, AND CLINICAL DECISION MAKING. Du Bois Review : Social Science Research on Race, 8(1), 199–218. https://doi.org/10.1017/S1742058X11000191
Vera-Cruz, A. (2022). Anti-HIV Activity of the Human Antimicrobial Peptide LL-37, and its Engineered Peptide, 17BIPHE2 [Thesis, Université d’Ottawa / University of Ottawa]. https://doi.org/10.20381/ruor-27605
Verlenden, J. V., Bertolli, J., & Warner, L. (2019). Contraceptive Practices and Reproductive Health Considerations for Adolescent and Adult Women with Intellectual and Developmental Disabilities: A Review of the Literature. Sexuality and Disability, 37(4), 541–557. https://doi.org/10.1007/s11195-019-09600-8
Walker, A. W., Stern, L., Cipres, D., Rodriguez, A., Alvarez, J., & Seidman, D. (2019). Do Adolescent Women’s Contraceptive Preferences Predict Method Use and Satisfaction? A Survey of Northern California Family Planning Clients. Journal of Adolescent Health,
64(5), 640–647. https://doi.org/10.1016/j.jadohealth.2018.10.291
Weber, L., & Malhi, S. (2024, March 21). Women are getting off birth control amid misinformation explosion. Washington Post. https://www.washingtonpost.com/health/2024/03/21/stopping-birth-control- misinformation/
Weinberger, M., Reidy, M., & Winfrey, W. (2021). Quantifying the potential market for new contraceptive technologies: Global projections of 2040 contraceptive needs and preferences. Gates Open Research, 5, 152. https://doi.org/10.12688/gatesopenres.13400.1
Weitzel, M. B. (2019, March 12). Yaso Therapeutics awarded $2 million SBIR grant from the National Institutes of Health to complete preclinical studies of a new female contraceptive that prevents disease transmission. Yaso Therapeutics. https://yasotherapeutics.com/yaso-therapeutics-awarded-2-million-sbir-grant-from- the-national-institutes-of-health-to-complete-preclinical-studies-of-a-new-female- contraceptive-that-prevents-disease-transmission/
White, K., Teal, S. B., & Potter, J. E. (2015). Contraception After Delivery and Short Interpregnancy Intervals Among Women in the United States. Obstetrics and Gynecology, 125(6), 1471–1477. https://doi.org/10.1097/AOG.0000000000000841
WHO. (2015). Medical Eligibility Criteria for Contraceptive Use, 5th edition. In Medical Eligibility Criteria for Contraceptive Use. 5th edition. World Health Organization. https://www.ncbi.nlm.nih.gov/books/NBK321153/
Wildemeersch, D., Jandi, S., Pett, A., Nolte, K., Hasskamp, T., & Vrijens, M. (2014). Use of frameless intrauterine devices and systems in young nulliparous and adolescent women: Results of a multicenter study. International Journal of Women’s Health, 6, 727–734. https://doi.org/10.2147/IJWH.S65462
Woodsong, C., & Alleman, P. (2008). Sexual pleasure, gender power, and microbicide acceptability in Zimbabwe and Malawi. AIDS Education and Prevention, 20(2), 171–187.
Woodsong, C., Shedlin, M., & Koo, H. (2004). The ‘natural’ body, God and contraceptive use in the southeastern United States. Culture, Health & Sexuality, 6(1), 61–78. https://doi.org/10.1080/13691050310001611165
World Professional Association for Transgender Health (WPATH). (2012). Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People (7th Version). World Professional Association for Transgender Health (WPATH). https://www.wpath.org/media/cms/Documents/SOC%20v7/SOC%20V7_English2012.pd f?_t=1613669341
Worly, B. L., Gur, T. L., & Schaffir, J. (2018). The relationship between progestin hormonal contraception and depression: A systematic review. Contraception, 97(6), 478–489. https://doi.org/10.1016/j.contraception.2018.01.010
Wouters, O. J., McKee, M., & Luyten, J. (2020). Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA, 323(9), 844– 853. https://doi.org/10.1001/jama.2020.1166
Wu, J., Meldrum, S., Dozier, A., Stanwood, N., & Fiscella, K. (2008). Contraceptive nonuse among US women at risk for unplanned pregnancy. Contraception, 78(4), 284–289. https://doi.org/10.1016/j.contraception.2008.04.124
Wu, J. P., McKee, K. S., McKee, M. M., Meade, M. A., Plegue, M. A., & Sen, A. (2017). Use of Reversible Contraceptive Methods Among U.S. Women with Physical or Sensory Disabilities. Perspectives on Sexual and Reproductive Health, 49(3), 141–147. https://doi.org/10.1363/psrh.12031
Xu, H., Eisenberg, D. L., Madden, T., Secura, G. M., & Peipert, J. F. (2014). Medical contraindications in women seeking combined hormonal contraception. American Journal of Obstetrics and Gynecology, 210(3), 210.e1-5. https://doi.org/10.1016/j.ajog.2013.11.023
Yavuz, B., Chambre, L., Harrington, K., Kluge, J., Valenti, L., & Kaplan, D. L. (2020). Silk Fibroin Microneedle Patches for the Sustained Release of Levonorgestrel. ACS Applied Bio Materials, 3(8), 5375–5382. https://doi.org/10.1021/acsabm.0c00671
Yee, L. M., & Simon, M. (2010). The role of the social network in contraceptive decision-making among young, African American and Latina women. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 47(4), 374–380.
https://doi.org/10.1016/j.jadohealth.2010.03.014
Yeh, P. T., Kautsar, H., Kennedy, C. E., & Gaffield, M. E. (2022). Values and preferences for
contraception: A global systematic review. Contraception, 111, 3–21. https://doi.org/10.1016/j.contraception.2022.04.011
YourChoice Therapeutics. (2023, November 12). YourChoice Therapeutics. YourChoice Bio.
https://www.yourchoicetx.com
Yuen, F., Thirumalai, A., Pham, C., Swerdloff, R. S., Anawalt, B. D., Liu, P. Y., Amory, J. K., Bremner, W. J., Dart, C., Wu, H., Hull, L., Blithe, D. L., Long, J., Wang, C., & Page, S. T. (2020). Daily Oral Administration of the Novel Androgen 11β-MNTDC Markedly Suppresses Serum Gonadotropins in Healthy Men. The Journal of Clinical Endocrinology and Metabolism, 105(3), e835-847. https://doi.org/10.1210/clinem/dgaa032
ZabBio. (2023). ZabBio. https://www.zabbio.com
Zaneva, M., Philpott, A., Singh, A., Larsson, G., & Gonsalves, L. (2022). What is the added value of incorporating pleasure in sexual health interventions? A systematic review and meta-analysis. PLOS ONE, 17(2), e0261034. https://doi.org/10.1371/journal.pone.0261034
Zhao, X., Milford, C., Smit, J., Zulu, B., Boyd, P., Malcolm, R. K., & Beksinska, M. (2022). Color, Scent and Size: Exploring Women’s Preferences Around Design Characteristics of Drug- Releasing Vaginal Rings. AIDS and Behavior, 1–15. https://doi.org/10.1007/s10461-022- 03596-7